Abstract
A reasonable gram quantity of the crystalline red dye 7-phenylbenzo[a]phenazine-5(7H)-one (PBP)was synthesised by the condensation of N-phenylbezene-1,2-diamine with 2-hydroxynaphthalene-1,4-dione in acetic acid (58% yield). The molecular structure of the dye, as determined by single-crystal X-ray crystallography, reveals a near planar phenazinone-like core, and an N-phenyl group twisted out of this plane by around 85°. The CO bond length of 1.241(2) Å is consistent with double bond character, which supports minor ground state zwitterionic character to the compound. The wavelength maximum for the observed partially structured low-energy absorption band is relatively insensitive to changes in the solvent polarity and polarizability. TD-DFT calculations predict that the long wavelength absorption envelope originates from localised π-π* transitions with no contribution from an n-π* state. The fluorescence quantum yield and singlet lifetime of the dye in MeCN are 0.12 and 3.0 ns, respectively. Fluorescence maxima are slightly sensitive to the solvent and changes in the Stokes shifts for a small series of alkanols were fitted to the Lippert-Mataga equation to afford a change in dipole moment of 8 ± 2 D. Calculations also reveal that full rotation of the N-phenyl group is severely restricted in the ground state (ΔEGS = 29 kcal mol−1) and in the first-excited singlet state (ΔEES = 34 kcal mol−1). The rocking back and forth of the phenyl group distorts the phenazinone-like backbone as it becomes co-planar and a minor solvent viscosity effect was observed in hydrogen bonding alkanol solvents.
Similar content being viewed by others
References
S. Hayashi, T. Takamatsu and S. Fujita, Cytofluorometric nuclear-DNA determinations on the atrioventricular nodel cells in human hearts, Histochemistry, 1986, 85, 111.
L. Michaelis, Rosinduline as oxidation-reduction indicator, J. Biol. Chem., 1931, 91, 369.
S. Abbasi, H. Barzegaramiri and A. Farmany, Determination of trace amounts of silver(I) in the presence of an activator with a kinetic method, Rare Met., 2014, 33, 731; H.-Y. Wang, Special analysis of dissolved copper in wastewater with azocarmine B by light absorption ratio variation combined with continuous analysis, J. Chin. Chem. Soc., 2008, 55, 1338.
O. Fischer and E. Hepp, The induline group, Liebigs Ann. Chem., 1891, 262, 237.
T. Xu, Y. Liu, F. Ge and Y. Ouyang, Application of surface methodology for optimization of azocarmine B removal by photo-Fenton process using hydroxyl-iron-aluminium bentonite, Appl. Surf. Sci., 2013, 280, 926.
T. Xu, Y. Liu, F. Ge and Y. Ouyang, Simulated solar light photooxidation of azocarmine B over hydroxyl iron-alu-minium pillared bentonite, Appl. Clay Sci., 2014, 100, 35.
G. Dormán, H. Nakamura, A. Pulsipher and G. D. Prestwich, The life of pi-star: Exploring the exciting and forbidden worlds of the benzophenone photophore, Chem. Rev., 2016, 116, 15284.
G. Dormán and G. D. Prestwich, Benzophenone photophores in biochemistry, Biochemistry, 1994, 33, 5661.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013.
M. A. El-Sayed, Triplet state. Its radiative and non radiative properties, Acc. Chem. Res., 1968, 1, 8.
R. Englman and J. Jortner, Energy gap law for radiationless transitions in large molecules, Mol. Phys., 1970, 18, 145.
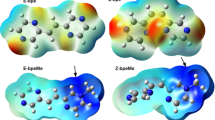
The hysteresis and shoulder seen in the figure may be explained by the fact that at the point where the N-phenyl group adopts a pyramidal geometry it can point up or down. The bowing of the structure need not be identical for the two cases leading to a loss of mirror symmetry between the two conformations. Semi-empirical calculations (AM1) performed on the ground- and excited-state structures showed the same effect albeit with slightly different energies to the rotation barriers (see ESIf). The barrier to rotation in the excited state was still around 5 kcal mol−1 larger than in the ground state and identical to the value from the DFT calculations. There are limitations to the method of using a single-coordinate in driving the energy minimization at each step change. The energy minimized structure may get trapped in a valley on the potential energy surface and not be able to “slip” to another structure. It is noted that the shoulder observed is not reproduced in a simple MM+ calculation, but the barrier to rotation is still significant (24 kcal mol−1).
E. Azanli, R. Rothchild and A.-M. Sapse, Ab-initio studies of hindered aryl rotations of methaqualone, mecloqualone and 3-(2,6-difluorophenyl)-2-methyl-4(3H)-quinazolinone, Spectrosc. Lett., 2002, 35, 257.
R. A. Loutfy and B. A. Arnold, Effect of viscosity and temperature on torsional relaxation of molecular rotors, J. Phys. Chem., 1982, 86, 4205.
J. Conradie, A frontier orbital energy approach to redox potentials, J. Phys.: Conf. Ser., 2015, 633, 012045.
I. B. Berlman, On an empirical correlation between nuclear conformation and certain fluorescence and absorption characteristics of aromatic compounds, J. Phys. Chem., 1970, 74, 3085.
S. J. Strickler and R. A. Berg, Relationship between absorption intensity and fluorescence lifetime of molecules, J. Chem. Phys., 1962, 37, 814.
At 80 K in EtOH the ϕFLU is ca. 60% meaning the quantum yield of intersystem crossing (ϕISC) plus the quantum yield for internal conversion (ϕIC) is about 40%. From comparison of the fluorescence area to the phosphorescence area at 80 K the ϕISC is only at best 1%.
T. Förster and G. Hoffmann, Viscosity dependence of fluorescent quantum yields of some dye systems, Z. Phys. Chem., 1971, 75, 63.
G. B. Dutt, Rotational dynamics of nondipolar probes in alkane-alkanol mixtures: Microscopic friction on hydrogen bonding and nonhydrogen bonding solute molecules, J. Chem. Phys., 2000, 113, 11154.
E. Z. Lippert, Dipolmoment und electronenstruktur von angeregten molekülen, Naturforscher, 1955, 10a, 541; N. Mataga, Y. Kaifu and M. Koizumi, Solvent effects upon fluorescence spectra and the dipole moments of excited molecules, Bull. Chem. Soc.Jpn., 1956, 29, 465.
The equation is more commonly written containing several terms including Planck's constant (h), speed of light (c) and the permittivity of free space (ε0). The terms and corrections for the units are all collected in the 10 068 number for simplicity. The equation can also be rewritten as where m is the slope of the Lippert-Mataga plot (see ref. 21).
A. R. Maryott and E. R. Smith, Table of dielectric constants of pure liquids, U.S. Govt. Print. Off., 1951.
A. M. Brouwer, Standards of photoluminescence quantum yields in solution, Pure Appl. Chem., 2011, 83, 2213.
R. C. Clark and J. S. Reid, The analytical calculation of absorption in multifaceted crystals, Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, 51, 887.
G. M. Sheldrick, SHELXT-integrated space-group and crystal structure determination, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3.
G. M. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112.
Acknowledgements
We thank the EPSRC sponsored mass spectrometry service at Swansea for collecting mass spectra and Newcastle University for financial support. Dr Joshua Karlsson is thanked for help in the nanosecond flash photolysis experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirbu, D., Wales, R., Geary, D.R. et al. Rosindone revisited: a computational and photophysical study of 7-phenylbenzo[a] phenazine-5(7H)-one (PBP). Photochem Photobiol Sci 18, 140–147 (2019). https://doi.org/10.1039/c8pp00279g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00279g