Abstract
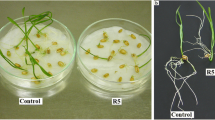
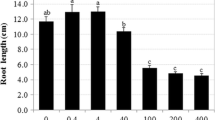
Inhibition of “Calypso” cucumber seedling growth by rye allelochemicals, 2(3H)-benzoxazolinone BOA and 2,4-dihydroxy-1,4(2H)-benzo- xazin-3-one DIBOA, was studied by analyzing the growth of seedling tissues and organs. Light and electron microscopy of seedling root cells were also carried out to investigate the mechanism(s) of root growth inhibition and mode of action of these compounds. BOA inhibited root elongation and reduced the number of cucumber lateral roots by 77 and 100% at 0.1 and 0.43 mg BOA/mlDeionized (DI) water, respectively. DIBOA also inhibited root growth, but did not affect the number of lateral roots. BOA increased size of cucumber cortical root cells fivefold, but DIBOA had no effect. Both compounds reduced the regeneration of root cap cells and increased the width of cortical cells resulting in increased root diameter. BOA and DIBOA caused increased cytoplasmic vacuolation, reduced ribosomeDensity and dictyosomes, reduced number of mitochondria, and reduced lipid catabolism. Starch granules in amyloplasts of seedling roots treated with BOA and DIBOA were also greatly reduced compared to the control. Changes in cellular ultrastructure indicated that BOA and DIBOA reduced root growth by disrupting lipid metabolism, reducing protein synthesis, and reducing transport or secretory capabilities.
Similar content being viewed by others
References
Aliotta, G., Cafiero, G., Florentino, A., and Strumia, S. 1993. Inhibition of radish germination and root growth by coumarin and phenylpropanoids. J. Chem. Ecol. 19:175–183.
Alsop, D. W. 1974. Rapid single-solution polychrome staining of semi-thin epoxy sections using polyethylene glycol 200 (PEG 200) as a stain solvent. Stain Technol. 49:265–272.
Barlow, P. W., Hawes, C. R., and Horne, J. C. 1984. Structure of amyloplasts and endoplasmic reticulum in the root caps of Lepidium sativum and Zea mays observed after selective membrane staining and by high-voltage electron microscopy. Planta 160:363–371.
Barnes, J. P. and Putnam, A. R. 1983. Rye residues contribute to weed suppression in no-tillage systems. J. Chem. Ecol. 9:1045–1057.
Barnes, J. P. and Putnam, A. R. 1987. Role of benzoxazinones in allelopathy by rye (Secale cereale L.). J. Chem. Ecol. 13:889–905.
Barnes, J. P., Putnam, A. R., Burke, B. A., and Aasen, A. J. 1987. Isolation and characterization of allelochemicals in rye herbage. Phytochemistry 26:1385–1390.
Burgos, N. R. and Talbert, R. E. 1996. Weed control and sweet corn (Zea mays var. rugosa) response in a no-till system with cover crops. Weed Sci. 44:355–361.
Burgos, N. R. and Talbert, R. E. 2000. Differential activity of allelochemicals from Secale cereale in seedling bioassays. Weed Sci. 48:302–310.
Burgos, N. R., Talbert, R. E., and Mattice, J. D. 1999. Cultivar and age differences in the production of allelochemicals by Secale cereale. Weed Sci. 47:481–485.
Cameron, H. J. and Julian, G. R. 1980. Inhibition of protein synthesis in lettuce (Lactuca sativa L.) by allelopathic compounds. J. Chem. Ecol. 6:989–995.
Chase, W. R., Nair, M. G., and Putnam, A. R. 1991. 2,2'-Oxo-1,1'-azobenzene-selective toxicity of rye (Secale cereale L.) allelochemicals to weed and crop species: II. J. Chem. Ecol. 17:9–19.
Einhellig, F. A., Muth, M. S., and Schon, M. K. 1985. Effects of allelochemicals on plant-water relationships, pp. 170–195, in A. C. Thompson (ed.). The Chemistry of Allelopathy. American Chemical Society, Washington, DC.
Einhellig, F. A. and Rasmussen, J. A. 1979. Effects of three phenolic acids on chlorophyll content and growth of soybean and grain sorghum seedlings. J. Chem. Ecol. 5:815–821.
Friebe, A., Roth, U., Kuck, P., Schnabl, H., and Schulz, M. 1997. Effects of 2,4-dihydroxy-1,4-benzoxazin-3-ones on the activity of plasma membrane H+-ATPase. Phytochemistry. 44:979–983.
Glass, A. D. M. 1973. Influence of phenolic acids on ion uptake I. Inhibition of phosphate uptake. Plant Physiol. 51:1037–1041.
Glass, A. D. M. and Dunlop, J. 1974. Influence of phenolic acids on ion uptake IV: Depolarization of membrane potentials. Plant Physiol. 54:855–858.
Gonzales, L. F. and Rojas, M. C. 1999. Role of wall peroxidases in oat growth inhibition by DIMBOA. Phytochemistry. 50:931–937.
Harper, J. R. and Balke, N. E. 1981. Characterization of the inhibition of K+ absorption in oat roots by salicylic acid. Plant Physiol. 68:1349–1353.
Hoshi, S. M., Usui, K., Ishizuka, K., Kosemura, S., Yamamura, S., and Hasegawa, K. 1994. Structure activity relationships of benzoxazolinones with respect to auxin-induced growth and auxin-binding protein. Phytochemistry. 37:297–300.
Jankay, P. and Muller, W. H. 1976. The relationships among umbelliferone, growth, and peroxidase levels in cucumber roots. Am. J. Bot. 63:126–132.
Karnovsky, M. J. 1965. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 27:137A–138A.
Kupidlowska, E., Kowalec, M., Sulkowski, G., and Zobel, A. M. 1994. The effect of coumarins on the root elongation and ultrastructure of meristematic cell protoplast. Ann. Bot. 73:525–530.
Liu, D. L. and Lovett, J. V. 1993. Biologically active secondary metabolites of barley. II. Phytotoxicity of barley allelochemicals. J. Chem. Ecol. 19:2231–2244.
Massardo, F., Zuniga, G. E., Perez, L. M., and Corcuera, L. J. 1994. Effects of hydroxamic acids on electron transport and their cellular location in corn. Phytochemistry. 35:873–876.
Mauseth, J. D. 1988. Plant Anatomy. Benjamin/Cummings, CA, USA, 560p.
Mollenhauer, H. H. 1974. Poststaining sections for electron microscopy. Stain Technol. 49:305–308.
Nair, M. G., Whitenack, C. J., and Putnam, A. R. 1990. 2,2'-Oxo-1,1'-azobenzene: A microbially transformed allelochemical from 2,3-benzoxazolinone. J. Chem. Ecol. 16:353–364.
Queirolo, C. B., Andreo, C. S., Niemeyer, H. M., and Corcuera, L. J. 1983. Inhibition of ATPase from chloroplasts by a hydroxamic acid from gramineae. Phytochemistry. 22:2455–2458.
Shilling, D. G., Liebl, R. A., and Worsham, A. D. 1985. Rye (Secale cereale L.) and wheat (Triticum aestivum L.) mulch: The suppression of certain broadleaf weeds and the isolation and identification of phytotoxins, pp. 243–271, in A. C. Thompson (ed.) The Chemistry of Allelopathy: Biochemical Interactions Among Plants. American Chemical Society, Washington, DC.
Spurr, A. R. 1969. A low-viscosity epoxy used in embedding medium for electron microscopy. J. Ultrastr. Res. 26:31–43.
Statistical Analysis System “SAS”. 1998. SAS/STAT User's Guide. Version 7. Electronic version. Statistical Analysis System Institute, Cary, NC.
Stenlid, G. 1970. Flavonoids as inhibitors of the formation of adenosine triphosphate in plant mitochondria. Phytochemistry. 9:2251–2256.
Warmke, H. E. and Lee, S. J. 1976. Improved staining procedures for semithin epoxy sections of plant tissues. Stain Technol. 51:179–185.
Weston, L. A. 1990. Cover crop and herbicide influence on row crop seedlingEstablishment in no-tillage culture. Weed Sci. 38:166–171.
Wilkes, M. A., Marshall, D. R., and Copeland, L. 1999. Hydroxamic acids in cereal roots inhibit the growth of take-all. Soil Biol. Biochem. 31:1831–1836.
Yenish, J. P., Worsham, A. D., and Chilton, W. S. 1995. Disappearance of DIBOA-glucoside, DIBOA, and BOA from rye (Secale cereale L.) cover crop residue. Weed Sci. 43:18–20.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burgos, N.R., Talbert, R.E., Kim, K.S. et al. Growth Inhibition and Root Ultrastructure of Cucumber Seedlings Exposed to Allelochemicals from Rye (Secale cereale). J Chem Ecol 30, 671–689 (2004). https://doi.org/10.1023/B:JOEC.0000018637.94002.ba
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000018637.94002.ba