Abstract
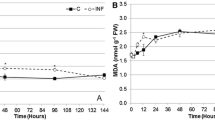
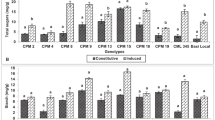
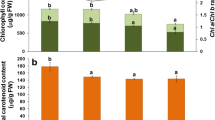
Induced resistance in cotton (Gossypium hirsutum) foliage and squares to herbivory by Helicoverpa zea (Lepidoptera: Noctuidae) is reported in this study. Induced resistance was indicated by decreased larval growth when larvae fed on previously damaged foliage or squares compared to the controls. Herbivory caused a significant decline in host nutritional quality as shown by a reduction in protein and most amino acids in both foliage and squares. Peroxidase, ascorbate oxidase, and diamine oxidase activities increased in both damaged foliage and squares, whereas levels of the nutritional antioxidant, ascorbate, were depressed after larval feeding. Larval feeding also markedly enhanced lipoxygenase activity and lipid peroxides in square tissues. Moreover, feeding damage altered the quantitative levels of phenolic compounds in foliage and squares. These results indicate a significant shift in the oxidative status of cotton plants following herbivory as indicated by increased oxidative enzyme activity, decreased levels of the nutritional antioxidant ascorbate, and increased levels of phenolic prooxidants (i.e., chlorogenic acid) and lipid peroxides.
Similar content being viewed by others
REFERENCES
AHMAD, S. 1992. Biochemical defense of pro-oxidant plant allelochemicals by herbivorous insects. Biochem Syst. Ecol. 20:269–296.
ANGELINI, R., and FEDERICO, R. 1989. Histochemical evidence of polyamine oxidation and generation of hydrogen peroxide in the cell wall. J. Plant Physiol. 135:212–217.
ANGELINI, R., MAINES, F., and FEDERICO, R. 1990. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon deetiolation and wounding in chick-pea stems. Planta 182:89–96.
AUERBACH, B. J., KIELY, J. S., and CORNICELLI, J. A. 1992. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal. Biochem. 201:375–380.
BARBER, M. S., and RIDE, J. P. 1988. A quantitative assay for induced lignification in wounded wheat leaves and its use to survey potential elicitors of the response. Physiol. Mol. Plant Pathol. 32:185–197.
BAYSAL, E., SULLIVAN, S. G., and STERN, A. 1989. Prooxidant and antioxidant effects of ascorbate on tBUOOH-induced electrolyte membrane damage. Int. J. Biochem. 21:113–138.
BELLES, J. M., TORNERO, P., CARBONELL, J., and CONEJERO, V. 1993. Polyamines in plant pathogenic signaling, pp. 160–164, in B. Fritig and M. Legrand (eds.). Mechanism of Plant Defense Responses. Kluwer Academic, Dordrecht/Boston/London.
BI, J. L., and FELTON, G. W. 1995. Foliar oxidative stress and insect herbivory: Primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 21:1511–1530.
BI, J. L., FELTON, G. W., and MUELLER, A. J. 1994. Induced resistance in soybean to Helicoverpa zea: Role of plant protein quality. J. Chem. Ecol. 20:183–198.
BRADFORD, O. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Chem. 72:248–254.
BRISSON, L. F., TENHAKEN, R., and LAMB, C. 1994. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6:1703–1712.
BRODY, A. K., and KARBAN, R. 1989. Demographic analysis of induced resistance against mites (Acari: Tetranychidae) in cotton. J. Econ. Entomol. 82:462–465.
CHAMULITRAT, W., HUGHES, M. F., ELING, T. E., and MASON, R. P. 1991. Superoxide and peroxyl radical generation from the reduction of polyunsaturated fatty acid hydroperoxides by soybean lipoxygenase. Arch. Biochem. Biophys. 290:153–159.
CHAN, B. G., WAISS, A. C., JR., BINDER, R. G., and ELLIGER, C. A. 1978a. Inhibition of lepidopterous larval growth by cotton constituents. Entomol. Exp. Appl. 24:94–100.
CHAN, B. G., WAISS, A. C., and LUKEFAHR, M. 1978b. Condensed tannins, an antibiotic chemical from Gossypium hirsutum. J. Insect Physiol. 24:113–118.
CHIOU, S. 1984. DNA-scission activities of ascorbate in the presence of metal chelates. J. Biochem. 96:1307–1310.
CHIPPENDALE, G. M. 1970. Metamorphic changes in fat body proteins of the southwestern corn borer Diatraea grandiosella. J. Insect Physiol. 16:1057–1068.
DUFFEY, S. S., and FELTON, G. W. 1989. Plant enzymes in resistance to insects, pp. 293–313, in J. R. Whitaker and P. E. Sonnet (eds.). Biocatalysis in Agricultural Biotechnology. American Chemical Society, Washington, DC.
DUFFEY, S. S., and FELTON, G. W. 1991. Enzymatic antinutritive defenses of tomato plants against insects, pp. 176–197, in P. A. Hedin (ed.). Naturally Occurring Pest Bioregulators. American Chemical Society, Washington, DC.
ELSTNER, E. F. 1987. Metabolism of activated oxygen species, pp. 253–315, in D. D. Davies (ed.). Biochemistry of Plants, Vol. 11, Academic Press, New York.
ENYEDI, A. J., YALPANI, N., SILVERMAN, P., and RASIN, I. 1992. Signal molecules in systemic plant resistance to pathogen and pest. Cell 70:879–886.
FEDERICO, R., and ANGELINI, R. 1986. Occurrence of diamine oxidase in the apoplast of pea epicotyls. Planta 167:300–303.
FEDERICO, R., and ANGELINI, R. 1988. Distribution of polyamines and their related catabolic enzyme in epicotyls of etiolated and light-grown Leguminosae seedlings. Planta 173:317–321.
FEDERICO, R., ANGELINI, R., CESTA, A., and PINI C. 1985. Determination of diamine oxidase in lentil seedlings by enzymatic activity and immunoreactivity. Plant Physiol. 79:62–64.
FELTON, G. W., and DUFFEY, S. S. 1991a. Protective action of midgut catalase in lepidopteran larvae against oxidative plant stress. J. Chem. Ecol. 17:1715–1732.
FELTON, G. W., and DUFFEY, S. S. 1991b. Reassessment of the role of gut alkalinity and detergency in insect herbivory. J. Chem. Ecol. 17:1821–1836.
FELTON, G. W., and SUMMERS, C. B. 1993. Potential role of ascorbate oxidase as a plant defense protein against insect herbivory. J. Chem. Ecol. 19:1553–1568.
FELTON, G. W., DONATO, K. K., DEL VECCHIO, R. J., and DUFFEY, S. S. 1989. Activation of plant foliar oxidases by insect feeding reduces the nutritive quality of foliage for noctuid herbivores. J. Chem. Ecol. 15:2667–2694.
FELTON, G. W., DONATO, K. K., BROADWAY, R. M., and DUFFEY, S. S. 1992. Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptea exigua. J. Insect Physiol. 38:277–285.
FELTON, G. W., SUMMERS, C. B., and MUELLER, A. J. 1994a. Oxidative responses in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa hopper. J. Chem. Ecol. 20:639–650.
FELTON, G. W., BI, J. L., MURELLER, A. J., and DUFFEY, S. S. 1994b. Potential role of lipoxygenases in defense against insect herbivory. J. Chem. Ecol. 20:651–666.
FRIDOVICH, I. 1978. The biology of oxygen radicals. Science 201:875–880.
GARA, L. D., TOMMASI, F., LISO, R., and ARROGINI, O. 1991. Ascorbic acid utilization by prolyl hydroxylase in vivo. Phytochemistry 30:1397–1399.
GASPAR, T., PREL, C., THORPE, T. A., and GREPPIN, H. 1981. Peroxidase, 1970–1981. University of Geneve Press, Geneva.
GRAYBURN, W. S., SCHNEIDER, G. R., HAMILTON-KEMP, T. R., BOOKJANS, G., ALI, K., and HILDEBRAND, D. F. 1991. Soybean leaves contain multiple lipoxygenases. Plant Physiol. 95:1214–1218.
GREELMAN, R. A., TIERNEY, M. L., and MULLET, J. E. 1992. Jasmonic and methyl jasmonate accumulate in wounded soybean and modulate wound gene expression. Proc. Natl. Acad. Sci. USA 89:4938–4941.
GUERRA, D. J. 1981. Natural and Heliothis zea-Induced Levels of Specific Phenolic Compounds in Gossypium hirsutum. Master's thesis. University of Arkansas, Fayetteville.
HAGERMAN, A. E., and BUTLER, L. 1991. Tannins and lignins, pp. 355–388, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores—Their Interactions with Secondary Plant Metabolites. Academic Press, New York.
HALLIWELL, B., and GUTTERIDGE, J. M. C. 1985. Free Radicals in Biology and Medicine. Clarendon Press, Oxford.
HALLIWELL, B., GROOTVELD, M., and GUTTERED, J. M. C. 1987. Methods for the measurement of hydroxyl radical in biochemical systems: Deoxyribose degradation and aromatic hydroxylation. Methods Biochem. Anal. 33:59–90.
HAMBERG, M., and GARDNER, H. W. 1992. Review—Oxylipin pathway to jasmonates: Biochemistry and biological significance. Biochim. Biophys. Acta 1165:1–18.
HATANAKA, A., KAJIMARA, T., and SEKIYA, J. 1987. Biosynthetic pathway for C6-aldehyde formation from linolenic acid in green leaves. Chem. Phys. Lipids 44:34–361.
HEDIN, P. A., JENKINS, J. N., COLLUM, D. H., WHITE, W. H., and PARROTT, W. L. 1983a. Multiple factors in cotton contributing to resistance to the tobacco budworm, Heliothis virescens F, pp. 347–365, in P. A. Hedin (ed.) Plant Resistance to Insects. American Chemical Society Symposium Series 208, Washington, DC.
HEDIN, P. A., JENKINS, J. N., COLLUM, D. H., WHITE, W. H., PARROTT, W. L., and MAC GOWN, M. W. 1983b. Cyanidin-3-β-glycoside, a newly recognized basis for resistance in cotton to the tobacco budworm Heliothis virescens (Fab.) (Lepidoptera: Noctuidae). Experientia 39:799–801.
HEDIN, P. A., JENKINS, J. N., and PARROTT, W. L. 1992. Evaluation of flavonoids in Gossypium arboreum (L.) cottons as potential source of resistance to tobacco budworm. J. Chem. Ecol. 18:105–114.
HU, M. L. 1994. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 233:380–385.
IMLAY, J. A., CHIN, S. M., and LINN, S. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in in vivo and in vitro. Science 240:640–642.
JACQ, B., LESOBRE, O., SANGWAN, R. S., and SANGWAN-NORREEL, B. S. 1993. Factors influencing tDNA transfer in Agrobacterium-mediated transformation of sugarbeet. Plant Cell Rep. 12:621–624.
KANOFSKY, J. R., and AXELROD, B. 1986. Singlet oxygen production by soybean lipoxygenase isozymes. J. Biol. Chem. 216:1099–1104.
KARBAN, R. 1986a. Induced resistance against spider mites in cotton: Field verification. Entomol. Exp. Appl. 42:239–242.
KARBAN, R. 1986b. Behavioural responses of spider mites (Tetranychus urticae) to induced resistance of cotton plants. Ecol. Entomol. 11:181–188.
KARBAN, R. 1987. Environmental conditions affecting the strength of induced resistance against mites in cotton. Oecologia 73: 414–419.
KARBAN, R. 1988. Resistance to beet armyworms (Spodoptera exigua) induced by exposure to spider mites (Tetranychus turkestani) in cotton. Am. Midl. Nat. 119:77–82.
KARBAN, R., and CAREY, J. R. 1983. Induced resistance of cotton seedlings to mites (Tetranychus urticae, Tetranychus turkestani, Tetranychus pacificus). Science 225:53–54.
KARBAN, R., and NIIHO, C. 1995. Induced resistance and susceptibility to herbivory: Plant memory and altered plant development. Ecology 76:1220–1225.
LAW, M. Y., CHARLES, S. A., and HALLIWELL, B. 1983. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplast. The effect of hydrogen peroxide and of paraquat. Biochem. J. 210:889–903.
LEGE, K. E., COTHREN, J. T., and SMITH, C. W. 1995. Phenolic acid and condensed tannin concentrations of six cotton genotypes. Environ. Exp. Bot. 35:241–249.
MACK, A. J., PETERMAN, T. K., and SIEDOW, J. N. 1987. Lipoxygenase isozymes in higher plants: Biochemical properties and physiological role. Curr. Topics Biol. Med. Res. 13:127–154.
MAKKAR, H. P. S., and BECKER, K. 1993. Vanillin-HCl method for condensed tannins: Effect of organic solvents used for extraction of tannins. J. Chem. Ecol. 19:613–621.
MAYER, A. M. 1987. Polyphenol oxidases in plants—Recent progress. Phytochemistry 26:11–20.
MURPHY, J. B., and STUTTE, C. A. 1978. Analysis for substituted benzoic and cinnamic acids using high-pressure liquid chromatography. Anal. Biochem. 86:220–228.
OSBORNE, D. J. 1978. Ethylene, pp. 265–294, in D. S. Letham, P. B. Goodwin, and T. J. V. Higgins (eds.). Phytohormones and Related Compounds—A Comprehensive Treatise. Elsevier/North-Holland Biomedical Press, Amsterdam/Oxford/New York.
PASCAL, R. G. 1972. Plant Phenolics. Hafner, New York.
PIERPOINT, W. S. 1983. Reactions of phenolic compounds with proteins, and their relevance to production of leaf proteins, pp. 235–267, in L. Telek and H. Graham (eds.). Leaf Protein Concentrates. AVI Publishing Company, Inc., Westport, CT.
REESE, J. C., CHAN, G. C., and WAISS, A. C., JR. 1982. Effect of cotton condensed tannin, maysin (cotton) and pinitol (soybeans) on Heliothis zea growth and development. J. Chem. Ecol. 8:1429–1437.
RIDGE, I., and OSBORNE, D. A. 1970. Hydroxyproline and peroxidases in cell walls of Pisum sativum: Regulation by ethylene. J. Exp. Bot. 21:843–856.
RYAN, J. D., GREGORY, P., and TINGEY, W. M. 1982. Phenolic oxidase activities in glandular trichomes of Solanum berthaultii. Phytochemistry 21:1885–1887.
SAS INSTITUTE. 1988. SAS/SAT User's Guide, 6.03 ed. SAS Institute, Cary, NC.
SCHEWE, T., REPORT, S. M., and KUHN, H. 1986. Entomology and physiology of reticulocyte lipoxygenase. Adv. Enzymol. Mol. Biol. 58:191–272.
SHIH, C. Y., DUMBROFF, E. B., and THOMPSON, J. E. 1989. Identification of a naturally occurring inhibitor of the conversion of l-aminocyclopropane-l-carboxylic acid to ethylene by carnation microsomes. Plant Physiol. 89:1053–1059.
SMITH, C. W., MC CARTY, J. C., JR., ALTAMARINO, T. P., LEGE, K. E., SCHUSTER, M. F., PHILIPS, J. R., and LOPEZ, J. D. 1992. Condensed tannins in cotton and bollworm-budworm (Lepidoptera: Noctuidae) resistance. J. Econ. Entomol. 85:2211–2217.
SMITH, T. A., and BAKER, J. H. A. 1988. The di-and polyamine oxidase of plants, pp. 573–587, in V. Zappia and A. E. Pegg (eds.). Progress in Polyamine Research. Plenum Press, New York.
SUMMERS, C. B., and FELTON, G. W. 1994. Prooxidant effect of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential mode of action of phenolic compounds in plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 24:943–953.
UCHIDA, K., ENOMOTO, N., ITAKURA, K., and KAWAKISHI, S. 1989. The hydroxyl radical generated by an ion (II)/EDTA/ascorbate system preferentially attacks tryptophan residues of the protein. Agr. Bio. Chem. 53:3285–3292.
UEDA, J., and KATO, J. 1980. Isolation and identification of a senescence-promoting substance from wormwood (Artemesia absinthium L.) Plant Physiol. 66:246–249.
VARNER, J. E., and BURTON, J. E. 1980. In vivo assay for synthesis of hydroxyproline-rich proteins. Plant. Physiol. 66:1044–1047.
BARNER, J. E., and LIN, L. S. 1989. Plant cell wall architecture. Cell 56:231–239.
VICK, B. A., and ZIMMERMAN, D. C. 1987. Oxidative systems for modification of fatty acids: the lipoxygenase pathway, pp. 53–90, in P. K. Stumpf (ed.). The Biochemistry of Plants—A Comprehensive Treatise, Vol. 9. Academic Press, Orlando, FL.
WOLFF, S. P. 1994. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxide. Methods Enzymol. 233:183–189.
ZUMMO, G. R., BENEDICT, J. H., and SEGERS, J. C. 1983. No-choice study of plant-insect interactions for Heliothis zea (Boddie) (Lepidoptera: Noctuidae) on selected cottons. Environ. Entomol. 12:1833–1836.
ZUMMO, G. R., SEGERS, J. C., and BENEDICT, J. H. 1984. Seasonal phenology of allelochemicals in cotton and resistance to bollworm (Lepidoptera: Noctuidae). Environ. Entomol. 13:1287–1290.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bi, J.L., Murphy, J.B. & Felton, G.W. Antinutritive and Oxidative Components as Mechanisms of Induced Resistance in Cotton to Helicoverpa zea . J Chem Ecol 23, 97–117 (1997). https://doi.org/10.1023/B:JOEC.0000006348.62578.fd
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000006348.62578.fd