Abstract
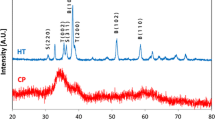
Ultrafine La0.7Sr0.3MnO3 powders were prepared via homogenization in chelate solutions, followed by the calcination of solid precursors at 700°C in air or oxygen, and their phase composition and average particle size were determined. The solid precursors were obtained from a solution of polynuclear La, Sr, and Mn chelates (diethylenetriaminepentaacetates) by three procedures: (1) gelation of the solution, followed by air drying of the resultant gel; (2) gelation followed by microwave dehydration; and (3) microwave dehydration of the solution. The results demonstrate that the way in which the chelate solution is converted into solid foams has little effect on the phase composition and particle size of the powders. At the same time, the phase composition and particle size of the reaction products depend on the calcination atmosphere (air or oxygen). Calcination in oxygen (700°C, 10 h) ensures the preparation of phase-pure La0.7Sr0.3MnO3 powders, with an average particle size of ≃30 nm, from the three precursors.
Similar content being viewed by others
REFERENCES
Jin, S., Tiefel, T., McCormac, M., et al., Thousandfold Change in Magnetoresistive La-Ca-Mn-O Films, Science, 1994, vol. 264, pp. 413–415.
Balcells, Ll., Martinez, B., Sandiumenge, F., and Fontcuberta, J., Magnetotransport Properties of Nanometric La2/3Sr1/3MnO3 Granular Perovskites, J. Magn. Magn. Mater., 2000, vol. 211, nos. 1–3, pp. 193–199.
Hwang, H., Cheong, S., Ong, N., and Batlogg, B., Spin-Polarized Intergrain Tunneling in La2/3Sr1/3MnO3, Phys. Rev. Lett., 1996, vol. 77, no. 10, pp. 2041–2046.
Tret'yakov, Yu.D., Tverdofaznye reaktsii (Solid-State Reactions), Moscow: Khimiya, 1978, pp. 123–125.
Metlin, Yu.G. and Tretyakov, Yu.D., Chemical Routes for Preparation of Oxide High-Temperature Superconducting Powders and Precursors for Superconductive Ceramics, Coatings, and Composites, J. Mater. Chem., 1994, vol. 4, no. 11, pp. 1659–1665.
Martynenko, L.I., Shlyakhtin, O.A., and Charkin, D.O., Synthesis of Barium Titanate from Chelates, Neorg. Mater., 1997, vol. 33, no. 5, pp. 581–592 [Inorg. Mater. (Engl. Transl.), vol. 33, no. 5, pp. 489–493].
Zhu, Y., Feng, J., Tan, R., et al., Preparation of Nanosized LaCoxMn1–xO3 Perovskite Oxide Using Amorphous Heteronuclear Complex as a Precursor, J. Alloys Compd., 2002, vol. 337, no. 1/2, pp. 282–288.
Zhu, Y., Tan, R., Yi, T., et al., Preparation of Nano-sized La2CuO4 Perovskite Oxide Using an Amorphous Heteronuclear Complex as a Precursor at Low-Temperature, J. Alloys Compd., 2000, vol. 311, no. 1, pp. 16–21.
Dedlovskaya, E.M., Kuz'mina, N.P., Antipov, A.B., et al., Synthesis of Ultrafine-Particle La0.7Sr0.3MnO3 via Chelate Homogenization and Microwave Processing, Neorg. Mater., 2002, vol. 38, no. 12, pp. 1499–1506 [Inorg. Mater. (Engl. Transl.), vol. 38, no. 12, pp. 1277–1283].
Sin, A., Synthesis of Mullite Powders by Acrylamide Polymerization, J. Mater. Sci. Lett., 2001, vol. 20, no. 17, pp. 1639–1641.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Antipov, A.B., Grigor'ev, A.N., Garshev, A.V. et al. Low-Temperature Synthesis of Ultrafine La0.7Sr0.3MnO3 Powder via Chelate Homogenization. Inorganic Materials 40, 661–665 (2004). https://doi.org/10.1023/B:INMA.0000032003.23763.a6
Issue Date:
DOI: https://doi.org/10.1023/B:INMA.0000032003.23763.a6