Abstract
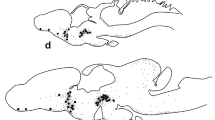
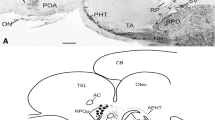
Leptin, its receptor and ACTH were detected by immunohistochemistry in the gastrointestinal tract and the neural tube of the amphibian urodele, Triturus cristatus carnifex, during development. These molecules were found after hatching of tadpoles, starting from stage 41. In the gastrointestinal tract, cells immunoreactive to leptin and its receptor were first revealed in the stomach, the liver and the gut and then in the pancreas. Both immunoreactives were colocalized in the same cells in some areas. Immunostaining for ACTH appeared at stages 43/45 in the stomach, the gut and the pancreas. In adjacent sections, a few cells immunoreactive to both ACTH and leptin receptor were detected. A few cells were immunoreactive to both insulin and leptin receptor. Immunoreactivities to leptin and its receptor were also found in adjacent sections of the neural tube, often colocalized in the same cell. Moreover, in prosencephalon, mesencephalon, rhomboencephalon and spinal cord, ACTH-immunoreactive cells were detected in the same areas as the leptin receptor immunoreactive cells. These results suggest the existence of a neuroendocrine network in newt tadpoles both at the central level, where it resembles that of mammals, and at the peripheral level, where it may act locally to regulate food intake and metabolism, e.g. yolk digestion.
Similar content being viewed by others
References
Bruni JF, Watkins WB, Yen SSC (1979) â-endorphin in the human pancreas. J Clin Endocrinol Metab 49: 649–651.
Buono S, Odierna G, Putti R (2001) Early stages of development of Triturus crisiatus pancreas. In: Goos HJTH, Rastogi RK, Vaudry H, Pierantoni R, eds. Perspective Comparative Endocrinology: Unity and Diversity. Bologna: Monduzzi Editore, pp. 189–195.
Cetin (1990) Immunohistochemistry of opioid peptides in the guinea pig endocrine pancreas. Cell Tissue Res 259: 313–319.
D'Aniello B, Imperatore C, Fiorentino M, Vallarino M, Rastogi RK (1994) Immunocytochemical localization of POMC-derived peptides (adrenocorticotropic hormone, á-melanocyte-stimulating hormone and â-endorphin) in pituitary, brain, and olfactory epithelium of the frog, Rana esculenta, during development. Cell Tissue Res 278: 509–516.
Della Rossa A, Putti R (1995) The endocrine pancreas of lacertids: An immunocytochemical study of the genera Pedioplanis and Meroles. Eur J Histochem 39: 47–58.
de Pablo F, Dashner R, Shuldiner AR, Roth J (1994) Xenopus laevis oocytes, eggs and tadpoles contain immunoactive insulin. J Endocrinol 141: 123–129.
El-Salhy M, Grimelius L (1981) Histochemical and immunohistochem-ical studies of the endocrine pancreas of lizards. Histochemistry 72: 237–247.
Emilsson V, O'Dowd J, Nolan AL, Cawthorne MA(2001) Hexosamines and nutrient excess induce leptin production and leptin receptor acti-vation in pancreatic islets and clonal beta-cells. Endocrinology 142: 4414–4419.
Ficele G, Heinig JA, Kawauchi H, Youson JH, Keeley FW, Wright GM (1998) Spatial and temporal distribution of Proopiomelanocortin and Proopicortin mRNAduring the life cycle of the sea lamprey: Aqualita-tive and quantitative in situ hybridization study. Gen Comp Endocrinol 110: 212–225.
Grube D, Voigt KH, Weber E (1978) Pancreatic glucagon cells contain endorphin-like immunoreactivity. Histochemistry 59: 75–79.
Guillemin R, Schally AV, Lipscomb HS, Andersen RN, Lung JM (1962) On the presence in hog hypothalamus of â-corticotropin releasing fac-tor, á and â-melanocyte stimulating hormones, adrenocorticotropin, lysine vasopressin and oxytocin. Endocrinology 70: 471–477.
Harland RM (1991) In situ hybridization: An improved whole-mount method for Xenopus embryos. Meth Cell Biol 36: 685–695.
Harrison RG (1969) Harrison stages and description of the normal devel-opment of the spotted salamander, Amblistoma punctatum (Linn). In: Harrison RG, ed. Organization and Development of the Embryo. New Haven: Yale University Press, pp. 44–66.
Horev G, Einat P, Aharoni T, Eshdat Y, Friedman-Einat M(2000) Molec-ular cloning and properties of the chicken leptin-receptor (CLEPR) gene. Mol Cell Endocrinol 162: 95–106.
Houseknecht KL, Baile CA, Matteri RL, Spurlock ME (1998) The biology of leptin: A review. J Anim Sci 76: 1305–1420.
Hummel A, Lendeckel U, Hahn von Dorsche H, Z¨uhlke H (1992) Pres-ence and regulation of a truncated proopiomelanocortin gene transcript in rat pancreatic islets. Biol Chem Hoppe-Seyler 373: 1039–1044.
Hummel A, Z¨uhlke H (1994) Expression of two Proopiomelanocortin mRNAs in the islets of Langerhans of neonatal rats. Biol Chem Hoppe-Seyler 375: 811–815.
Islam MS, Morton NM, Hansson A, Emilsson V (1997) Rat insulinoma-derived pancreatic â-cells express a functional leptin receptor that mediates a proliferative response. Biochem Biophys Res Commun 238: 852–855.
Islam MS, Sjohom A, Emilsson V (2000) Foetal pancreatic islets express functional leptin receptors and leptin stimulates proliferation of foetal islet cells. In J Obes Relat Metab Disord 24: 1246–1253.
J`egou S, Tonon MC, Leroux P, Delarue C, Leboulenger F, Pelletier G, Cot`e J, Long N, Vaudry H (1983) Immunological characteriza-tion of endophins, adrenocorticotropin, and melanotropins in frog hypothalamus. Gen Comp Endocrinol 51: 246–254.
Johnson RM, Johnson TM, Londraville RL (2000) Evidence for leptin expression in fishes. J Exp Zool 286: 718–724.
Kapcala LP (1985) Immunoassayable adrenocorticotropin in periph-eral organs: Concentrations during early development. Life Sci 37: 2283–2290.
Kask A, Skottner A (2001) Melanocortin receptors in the regulation of food intake. In: Goos HJTh, Rastogi RK, Vaudry H, Pierantoni R, eds. Perspective Comparative Endocrinology: Unity and Diversity. Bologna: Monduzzi Editore, pp. 677–682.
Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF (1997) Leptin suppression of insulin secretion by the activation of ATP-sensitive K +channels in pancreatic beta-cells. Diabetes 46: 1087–1093.
Krieger DT, Liotta A, Brownstein MJ (1977) Presence of corticotropin in brain of normal and hypophysectomized rats. Proc Natl Acad Sci USA 74: 648–652.
Larsson LI (1977) Corticotropin-like peptides in central nerves and in endocrine cells of gut and pancreas. Lancet ii: 1321–1323.
Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzade JG, Lee JI, Friedman JM (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635.
Lewin MJ, Bado A (2001) Gastric leptin. Microsc Res Tech 53: 372–376.
Lin J, Barb CR, Matteri RL, Kraeling RR, Chen X, Meinersmann RJ, Rampacek GB (2000) Long form leptin receptor mRNA expression in the brain, pit and other tissue in the pig. Domest Anim Endocrinol 19: 53–61.
Magni P, Motta M, Martini L (2000) Leptin: A possible link between food intake, energy expenditure, and reproductive function. Regulatory Peptides 92: 51–56..Leptin, leptin receptors and ACTH in newt tadpoles 109
Malendowicz LK, Neri G, Jedrzejczak N, Hochol A, Nussdorfer GG (2000) Effects of recombinant murine leptin [1–147] and leptin frag-ment 116–130 on steroid secretion and proliferative activity of the regenerating rat adrenal cortex. Endocr Res 26: 109–118.
Morash B, Johnstone J, Leopold C, Li A, Murphy P, Ur E, Wilkinson M (2000) The regulation of leptin gene expression in the C6 glioblastoma cell line. Mol Cell Endocrinol 165: 97–105.
Niendorf WR, Z¨uhlke H (1985) Biosynthesis of â-endorphin in pan-creatic islets of neonatal Wistar rats. Biomed Biochem Acta 44: K51–K54.
Paolucci M, Rocco M, Varricchio E (2001) Leptin presence in plasma, liver and fat bodies in the lizard Podarcis sicula fluctuations throughout the reproductive cycle. Life Sci 69: 2399–2408.
Putti R, Della Rossa A, Varano L, Laforgia V, Cavagnuolo A (1992) An immunocytochemical study of the endocrine pancreas in three genera of lacertids. Gen Comp Endocrinol 87: 249–259.
Putti R, Della Rossa A, Tagliafierro G, Maglio M(1995) Cell composition and co-stored peptides in the endocrine pancreas of Rana arvalis. Eur J Histochem 39: 59–68.
Putti R, Della Rossa A, Maglio M, Tagliafierro G (1997) Islets and dif-fuse endocrine component in the pancreas of three red frogs species. Relationships between endocrine and exocrine pancreas tissue. Tissue Cell 29: 355–363.
Putti R, Buono S, Ottaviani E (1999) PP/PYY cells from endocrine pancreas of the scincid lizard Eumeces inexpecatatus synthesize ACTH-and á-MSH-like molecules. Gen Comp Endocrinol 116: 153–163.
Sanchez-Franco F, Patel YC, Reichlin S (1981) Immunoreactive adreno-corticotropin in the gastrointestinal tract and pancreatic islets of the rat. Endocrinology 108: 2235–2238.
Steffens AB, Adage T, De Vries K, Adan R, Scheurink A, Van Dijk G (2001) Role of the leptin signaling system in energy homeostasis. In: Goos HJTH, Rastogi RK, Vaudry H, Pierantoni R, eds. Perspective Comparative Endocrinology: Unity and Diversity. Bologna: Monduzzi Editore, pp. 693–701.
Tanizawa Y, Okuya S, Ishihara H, Asano T, Yada T, Oka Y (1997) Direct stimulation of basal insulin secretion by physiological concentrations of leptin in pancreatic â cells. Endocrinology 138: 4513–4516.
Udagawa J, Hatta T, Naora H, Otani H (2000) Expression of the long form of leptin receptor (Ob-Rb) mRNA in the brain of mouse embryos and newborn mice. Brain Res 868: 251–258.
Volkoff H, Peter RE(2001) Novel peptides involved in the control of feedingin goldfish. In: Goos HJTH, Rastogi RK, Vaudry H, Pierantoni R,eds. Perspective Comparative Endocrinology: Unity and Diversity. Bologna: Monduzzi Editore, pp. 703–709.
Wauters M, Considine RV, Van Gaal LF (2000) Human leptin: From an adypocyte hormone to an endocrine mediator. Eur J Endocrinol 143: 293–311.
Yu NW, Hsu CY, Ku HH, Wang HC (1985) The development of ACTH-like substance during tadpole metamorphosis. Gen Comp Endocrinol 57: 72–76.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JH (1994) Positional cloning of the mouse obese gene and its human analogue. Nature 372: 425–432.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buono, S., Putti, R. Leptin, Leptin Receptors and ACTH Immunoreactivities are Present in the Gastrointestinal Tract and the Neural Tube of Tadpoles of the Newt Triturus . Histochem J 35, 103–109 (2004). https://doi.org/10.1023/B:HIJO.0000023370.08524.fc
Issue Date:
DOI: https://doi.org/10.1023/B:HIJO.0000023370.08524.fc