Abstract
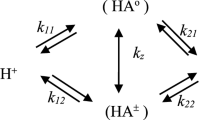
Theoretic fundamentals of the method of pK spectroscopy are formulated as applied to investigating acid–base properties of suspensions, hydrosols, and solutions of macromolecules. The computer-aided treatment of the data of potentiometric titration of the relevant substrates makes it possible to determine the molar fractions of corresponding acid–base groups (per total number of these groups) as a function of pK values of these groups. An appropriate computer program is tested with a model example and resulted in a pK spectrum meeting the model requirements. The method of pK spectroscopy is used to study acid–base properties of γ-Al2O3 suspension. In the pK spectrum obtained, four bands corresponding to four types of acid–base groups on the oxide surface are observed that appears to be a direct verification of the James–Parks model.
Similar content being viewed by others
REFERENCES
James, R.O. and Parks, G.A., Surface and Colloid Science, Matijevic, E.N., Ed., New York: Plenum, 1982, vol. 12, p. 116.
Golikov, A.P., Zh. Fiz. Khim., 1995, vol. 69, no. 4, p. 664.
Bratskaya, S.Yu. and Golikov, A.P., Zh. Anal. Khim., 1998, vol. 53, no. 3, p. 265.
Hunston, D.N., Anal. Biochem., 1975, vol. 63, no. 1, p. 99.
Leuenberger, B. and Schindler, P.W., Anal. Chem., 1986, vol. 58, no. 7, p. 1471.
Garmash, A.V. and Vorob'eva, O.N., Zh. Anal. Khim., 1998, vol. 53, no. 3, p. 258.
Ryazanov, M.A., Ryazanov, A.M., and Zlobin, D.A., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2000, vol. 43, no. 5, p. 150.
Simms, H.S., J. Am. Chem. Soc., 1926, vol. 48, no. 5, p. 1239.
Rusanov, A.I., Abstracts of Papers, VIII Mezhdunarodnaya konferentsiya “Vysokotemperaturnaya khimiya silikatov i oksidov” (VIII Int. Conf. “High-Temperature Chemistry of Silicates and Oxides”), St. Petersburg: OOPNIIKh St. Petersburg State University, 2002, p. 5.
Chan, D., Perram, J.W., White, L.B., and Healy, T.W., J. Chem. Soc., Faraday Trans. 1, 1975, vol. 71, no. 6, p. 1046.
Fridrikhsberg, D.A., Kurs kolloidnoi khimii (Handbook of Colloid Chemistry), Leningrad: Khimiya, 1974.
Shchukin, E.D., Pertsov, A.V., and Amelina, E.A., Kolloidnaya khimiya (Colloid Chemistry), Moscow: Mosk. Gos. Univ., 1982.
Lawson, C.L. and Hanson, R.J., Solving Least Squares Problems, Englewood Cliffs: Prentice-Hall, 1974.
Tikhonov, A.N. and Arsenin, V.Ya., Metody resheniya nekorrektnykh zadach (Solution of Ill-Posed Problems), Moscow: Nauka, 1986.
Dudkin, B.N., Kaneva, S.I., Mastikhin, V.M., and Pletnev, R.N., Zh. Prikl. Khim. (St. Petersburg), 2000, vol. 70, no. 12, p. 1949.
Hyang, Ch.P. and Stumm, W., J. Colloid Interface Sci., 1973, vol. 43, no. 2, p. 409.
Zhukov, A.N., Kolloidn. Zh., 1996, vol. 58, no. 2, p. 280.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ryazanov, M.A., Dudkin, B.N. Acid–Base Properties of γ-Al2O3 Suspension Studied by pK Spectroscopy. Colloid Journal 65, 761–766 (2003). https://doi.org/10.1023/B:COLL.0000009121.65691.24
Issue Date:
DOI: https://doi.org/10.1023/B:COLL.0000009121.65691.24