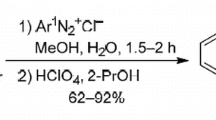
Abstract
Treatment of p-phenylenediamine with maleic acid and its diethyl ester gave di- and tetracarboxylic amino acids and their esters. A benzene derivative having an α-alanine and an aspartic acid residue has been prepared. The cyclization of aminocarboxylic acids to imidazole and pyrimidine derivatives has been carried out.
Similar content being viewed by others
REFERENCES
R. S. Baltrushis, Z.-I. G. Beresnevichyus, I. M. Vizgaitis, and Yu. V. Gatilov, Khim. Geterotsikl. Soedin., 1267 (1983).
R. S. Baltrushis, Z.-I. G. Beresnevichyus, I. M. Vizgaitis, and Yu. V. Gatilov, Khim. Geterotsikl. Soedin., 1669 (1981).
W. Reppe and H. Uffer, US Patent 2200220; Chem. Abstr., 34, 5859 (1940).
Z.-I. G. Beresnevicius, Doctorial Dissertation in Chemical Sciences, Kaunas (1989).
G. A. Makhteeva, Dissertation of Candidates in Chemical Sciences, Kaunas (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rutkauskas, K., Beresnevicius, ZI. Cyclization of the Reaction Products of p-Phenylenediamine with Maleic Acid. Chemistry of Heterocyclic Compounds 40, 792–796 (2004). https://doi.org/10.1023/B:COHC.0000040777.58344.5a
Issue Date:
DOI: https://doi.org/10.1023/B:COHC.0000040777.58344.5a