Abstract
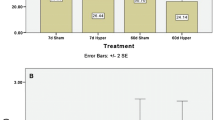
The present study was focused on the influence of mercury on the rat liver and kidney glucocorticoid receptor (GR) binding properties. The time-course and dose-dependence of mercury effects, as well as possible involvement of thiol groups were examined afterin vivo andin vitro administration of the metal in the form of HgCl2. Mercury led to reduction of the liver and kidney GR hormone binding capacity. In both examined tissues maximal reduction was noticed 4 h after administration of the metal at 2 and 3 mg Hg/kg bw, but the effect was more prominent in kidney as compared to liver. On the other hand, binding affinity in the two tissues was similar. The complete reversal of mercury effects on GR binding capacity by 10 mmol/L DTT was achieved in liver and partially in kidney. The reversal by DTT suggested that mercury caused the decrease of GR binding activity by interacting with thiol groups. The difference in the response of the two tissues reflected the fact that kidney contained a higher mercury concentration and a lower thiol content in comparison to liver. The implicated thiols probably belong to GR, since when appliedin vitro at 0°C, mercury produced reduction of the receptor binding activity similar to that observedin vivo. GR protein level examined by quantitative Western blot was either unchanged, when determined by polyclonal antibody, or reduced, when determined by BuGR2 antibody, suggesting that Hg might affect BuGR epitope availability.
Similar content being viewed by others
References
Agemian H, Chau ASY. A method for determination of mercury in sediments by the automated cold vapor atomic absorption technique after digestion. Anal Chim Acta. 1975;75:297–304.
Beato M, Feigelson P. Glucocorticoid binding proteins of rat liver cytosol. Separation and identification of binding proteins. J Biol Chem. 1972;247:7890–6.
Bodine PV, Litwack G. The glucocorticoid receptor and its endogenous regulators. In: Litwack G, ed. Receptor. Totowa: Humana Press, 1990:83–119.
Bodwell JE, Holbrook NJ, Munck A. Evidence for distinct sulfhydryl groups associated with steroid-and DNA-binding domains of rat thymus glucocorticoid receptors. Biochemistry. 1984;23:4237–42.
Chakraborti PK, Garabedian MJ, Yamamoto KR, Simons SS Jr. Role of cysteines 640, 656 and 661 in steroid binding to rat glucocorticoid receptors. J Biol Chem. 1992;267:11366–73.
Chowdhury BA, Chandra RK. Biological and health implications of toxic heavy metal and essential trace element interactions. Prog Fd Nutr Sci. 1987;11:55–113.
Coty WA. Reversible dissociation of steroid hormone-receptor complexes by mercurial reagents. J Biol Chem. 1980;255(17):8035–7.
Czar MJ, Owens-Grillo JK, Dittmar KD, et al. Characterization of the protein-protein interactions determining the heat shock protein (hsp 90.hsp 70.hsp 56) heterocomplex. J Biol Chem. 1994;269:11155–61.
DeMoor JM, Koropatnic JD. Metals and cellular signaling in mammalian cells. Cell Mol Biol. 2000;46(2):367–81.
Dundjerski J, Butorović B, Kipić J, Trajković D, Matić G. Cadmium and dexamethasone affect glucocorticoid receptor level and degradation in the rat liver. Arch Biol Sci. 1996;48:79–86.
Dundjerski J, Kovać T, Pavković N, Cvoro A, Matić G. Glucocorticoid receptor-Hsp90 interaction in the liver cytosol of cadmium-intoxicated rats. Cell Biol Toxicol. 2000a;16:375–83.
Dundjerski J, Predić J, Kovać T, et al. A possible role of metallothionein and heat shock proteins in glucocorticoid receptor protection against cadmium intoxication. Arch Biol Sci. 2000b;52:89–95.
Dundjerski J, Stanošević J, Ristić B, Trajković D, Matić G. In vivo effects of cadmium on rat liver glucocorticoid receptor functional properties. Int J Biochem. 1992;24:1065–72.
Goering PL, Fisher BR, Chaudhary PR, Dick CA. Relationship between stress protein induction in rat kidney by mercuric chloride and nephrotoxicity. Toxicol Appl Pharmacol. 1992;113:184–91.
Goyer R. Toxic effects of metals. In: Klassen CD, Amdur MO, Doull J, eds. Casarett and Doull's toxicology: the basic science of poisons, 5th edn. New York: McGraw-Hill. 1996:691–736.
Grandjean P. Diseases associated with metals. In: Last JM, ed. Public health and preventive medicine, 23rd edn. Norwalk, CT: Appleton-Century-Crofts. 1986:587–616.
Holmes E, Bonner FW, Sweatman BC, et al. Nuclear magnetic resonance spectroscopy and pattern recognition analysis of the biochemical processes associated with the progression and recovery of nephrotoxic lesions in the rat induced by mercury II chloride and 2-bromoethanamine. Mol Pharmacol. 1992;42:922–30.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Lind B, Friberg L, Nylander M. Preliminary studies on metilmercury biotransformation and clearance in the brain of primates: II. Demetilation of mercury in the brain. J Trace Elements Exp Med. 1988;1:49–56.
Meshinchi S, Matić G, Hutchinson KA, Pratt WB. Selective molybdate-directed covalent modification of sulfhydryl groups in the steroid-binding versus DNA-binding domain of the glucocorticoid receptor. J Biol Chem. 1990;256:11643–9.
Miesfeld R, Rusconi S, Godowski PJ, et al. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986;46:389–99.
Sanchez DJ, Belles M, Albina LM, Sirvent JJ, Domingo JL. Nephrotoxicity of simultaneous exposure to mercury and uranium in comparison to individual effects on these metals in rats. Biol Trace Element Res. 2001;84:139–54.
Sanchez Uria JE, Sanz-Medel. Inorganic and methylmercury speciation in environmental samples. Talanta. 1998;47:509–24.
Simons SS Jr. Function/activity of specific amino acids in glucocorticoid receptors. Vitamins Hormones. 1994;49:49–130.
Simons SS Jr, Chakraborti PK, Cavanaugh AH. Arsenite and cadmium (II) as probes of glucocorticoid receptor structure and function. J Biol Chem. 1990;265:1938–45.
Spector T. Refinement of the coomassie blue method of protein quantitation. Anal Biochem. 1978;86:142–6.
Stein ED, Cohen Y, Winer AM. Environmental distribution and transformation of mercury compounds, Crit Rev Environ Sci Technol. 1996;26:1–43.
Takahashi H, Suetomi K, Konishi T. Differential determination of ionizable and unionizable (inert) forms of inorganic mercury in animal tissues. In: Suzuki T, Imura N, Clarkson TW, eds. Advances in mercury toxicology. 19th Rochester International Conference on Environmental Toxicity. New York: Plenum Press. 1991:181–9.
Telford WG, Fraker PJ. Zinc reversibly inhibits steroid binding to murine glucocorticoid receptor. Biochem Biophys Res Comm. 1997;283:86–9.
Zalups RK, Gelein RM, Cernichiari E. DMPS as a rescue agent for the nephropathy induced by mercuric chloride. J Pharm Exp Ther. 1990;256:1–10.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brkljačić, J., Vojnović Milutinović, D., Dundjerski, J. et al. Mercury inhibits rat liver and kidney glucocorticoid receptor hormone binding activity. Cell Biol Toxicol 20, 171–182 (2004). https://doi.org/10.1023/B:CBTO.0000029467.21231.12
Issue Date:
DOI: https://doi.org/10.1023/B:CBTO.0000029467.21231.12