Abstract
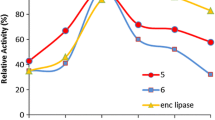
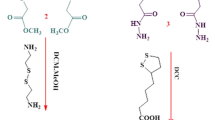
The enantioselective hydrolysis of insoluble (R,S)-ketoprofen ethyl ester to the optically active (S)-ketoprofen was carried out in a dispersed aqueous lipase reaction system induced by the inclusion of chiral cyclodextrins for complexation of the substrate. Hydroxypropyl-β-cyclodextrin was the most effective chiral selector and disperser giving an enantiomeric excess and conversion yield of 0.99 and 0.49, respectively.
Similar content being viewed by others
References
Ceynowa J, Rauchfleisz M (2003) High enantioselective resolution of racemic 2-aryl-propionic acids in an enzyme membrane reactor. J. Mol. Catal. B: Enzym. 23: 43–51.
Cipiciani A, Bellazza F, Fringuelli F, Stillitano M (1999) Enantioselectivity of alcohol-treated Candida rugosa lipase in the kinetic resolution of racemic methyl 2-aryloxy-propionates in water and aqueous organic media. Tetrahedron:Asymmetry 10: 4599–4605.
Ghanem A (2003) The utility of cyclodextrins in lipase-catalyzed transesterfications in organic solvents: enhanced reaction rate and enantioselectivity. Org. Biomol. Chem. 1: 1282–1291.
Jin JN, Lee SH, Lee SB (2003) Enzymatic production of enantiopure ketoprofen in a solvent-free two-phase system. J. Mol. Catal. B: Enzym. 26: 209–216.
KimMG, Lee EG, Chung BH (2000) Improved enantioselectivity of Candida rugosa lipase towards ketoprofen ethyl ester by a simple two-step treatment. Process Biochem. 35: 977–982.
Kwon DY, Rhee JS (1986) A simple and rapid colorimetric method for determination of free fatty acids for lipase assay. JAOCS 63: 89–91.
Lee YH, Jeong SH, Park DC (1995) Comparison of inclusion complex formation capacity of cyclodextrin with various molecules and characterization of cyclodextrin-fatty acid complex. Kor. J. Biotechnol. Bioeng. 10: 149–158.
Lee YH, Kim TK, Shin HD, Park DC (1998) Enzymatic hydrolysis of hydrophobic triolein by lipase in a mono-phase reaction system containing cyclodextrin: reaction characteristics. Biotechnol. Bioprocess Eng. 3: 103–108.
Liu YY, Xu JH, Hu Y (2000) Enhancing effect of tween-80 on lipase performance in enantioselectivity hydrolysis of ketoprofen ester. J. Mol. Catal. B: Enzym. 10: 523–529.
Manetti F, Mileto L, Corelli F, Soro S, Palocci C, Cernia E, D'Acquarica I, Lotti M, Alberghina L, Botta M (2000) Design and realization of a tailor-made enzyme to modify the molecular recognition of 2-arylpropionic esters by Candida rugosa lipase. Biochim. Biophys. Acta 1543: 146–158.
Mine Y, Fukunaga K, Itoh K, Yoshimoto M, Nakao K, Sugimura Y (2003) Enhanced enzyme activity and enantioselectivity of lipases in organic solvents by crown ethers and cyclodextrins. J. Biosci. Bioeng. 95: 441–447.
Mine Y, Fukunaga K, Yoshimoto M, Nakao K, Sugimura Y (2001) Modification of lipases with poly(ethyleneglycol) and poly(oxyethylene) detergent and their catalytic activities in organic solvents. J. Biosci. Bioeng. 92: 539–543.
Rekharsky MB, Inoue Y (1998) Complexation thermodynamics of cyclodextrins. Chem. Rev. 98: 1875–1918.
Shang CS, Hsu CS (2003) Lipase-catalyzed enantioselective esterification of (S)-naproxen hydroxyalkyl ester in organic media. Biotechnol. Lett. 25: 413–416.
Shin HD, Kim JH, Kim TK, Kim SH, Lee YH (2002) Esterification of hydrophobic substrates by lipase in the cyclodextrin induced emulsion reaction system. Enzyme Microb. Technol. 30: 835–842.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, SH., Kim, TK., Shin, GS. et al. Enantioselective hydrolysis of insoluble (R,S)-ketoprofen ethyl ester in dispersed aqueous reaction system induced by chiral cyclodextrin. Biotechnology Letters 26, 965–969 (2004). https://doi.org/10.1023/B:BILE.0000030040.13828.d7
Issue Date:
DOI: https://doi.org/10.1023/B:BILE.0000030040.13828.d7