Abstract
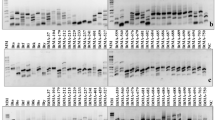
Polyacrylamide gel electrophoresis was used to analyze esterase patterns during development of Aedes aegypti from the cities of Marília and São José do Rio Preto (SJRP), Brazil. The zymograms showed a total of 23 esterase bands, 22 of which were in the specimens from Marília and 19 in those from SJRP. These esterase bands were considered to be the product of 23 alleles distributed tentatively in eight genetic loci. Most of the alleles were developmentally regulated. The larval stage expressed the greatest number of them (19 alleles, from the eight loci, in Marília; and 17 alleles, from seven loci, in SJRP). The pupal stage expressed 10 alleles from seven loci, in both populations, and the adult stage expressed 8 alleles from five and six loci in SJRP and Marília, respectively. Some alleles that were active in every stage were developmentally controlled at the level of expression (amount of product). A single allele was constitutively and highly expressed, in larvae, pupae, and adults, in both populations. Differences in esterase synthesis among stages are probably due to regulatory mechanisms acting in agreement with the requirements of a variable number of processes in which esterases are involved. The larval stage is the most active in developmental processes and shows very intense intake of food and very high mobility. These features may demand increased esterase production at that stage. Comparison of the two populations examined showed (besides the existence of alleles that they do not share) that they exhibit differences in the control of expression of other alleles. Such findings may reflect genetic differences between founders in each population, but the possibility of involvement of the intensive use of insecticides in SJRP is also discussed.
Similar content being viewed by others
REFERENCES
Aldridge, W. N. (1953). Serum esterases: Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem. J. 53:110–117.
Arnason, E., and Chambers, G. K. (1984). Substrate specificity of esterases in D. pseudoobscuraand D. melanogaster, with notes on the tissue localization of esterase-5 in D. pseudoobscura. Dros. Inf. Serv. 60:52–53.
Brady, J. P., and Richmond, R. C. (1990). Molecular analysis of evolutionary changes in the expression of Drosophilaesterases. Proc. Nat. Acad. Sci. U.S.A. 87:8217–8221.
Castiglioni-Ruiz, L., Bicudo, H. E. M. C., and Ceron, C. R. (1997). Esterase patterns in four Brazilian populations of Hematobia irritans. Cytobios 90:81–94.
Ceron, C. R., Santos, J. R., and Bicudo, H. E. M. C. (1992). The use of gelatin to dry cellophane wound slab gels in an embroidering hoop. Rev. Bras. Genét. 15:201–203.
Chen, Y. P., and Sudderunddin, K. I. (1978). Toxicological studies of insecticides on Culex quinquefasciatussay and Aedes aegypti(L). Southeast Asian J. Trop. Med. Public Health 9:378–383.
Cordeiro, J. A. (1987). Analysis of dependence, Relatório Técnico 48/87, Instituto de Matemática da Universidade Estadual de Campinas.
Dekort, C. A. D., and Granger, N. A. (1981). Regulation of the juvenile hormone titer. Annu. Rev. Entomol. 26:1–28.
Dufer, J., Trentesaux, C., and Desplaces, A. (1984). Differential effect of the serine protease inhibitor phenyl methyl sulphonyl fluoride on cytochemically detectable esterases in human leucocytes and platelets. Scand. J. Haematol. 32:25–32.
Field, W. M., and Hitchen, J. M. (1981). Linkage relationships between an esterase locus and group II markers in the yellow fever mosquito, Aedes aegypti(Diptera: Culicidae). J. Med. Entomol. 18:61–64.
Field, W. M., Hitchen, J. M., and Rees, A. T. (1984). Esterase activity in strains of Aedes aegypti(Diptera: Culicidae) tolerant and susceptible to the organophosphate insecticide Malathion. J. Med. Entomol. 21:412–418.
Fournier, D., Mutero, A., Pralavorio, M., and Bride, J. M. (1993). Drosophilaacetylcholinesterase: Mechanisms of resistance to organophosphates. Chem. Biol. Interact. 87:233–238.
Johnson, F. M., Kanapi, C. G., Richardson, R. H., Wheeler, M. R., and Stone, W. S. (1966). XVIII. An operational classification of Drosophilaesterases for species comparison. Univ. Texas Public. 6615:517–532.
Jones, B. R., and Brancoft, H. R. (1986). Distribution and probable physiological role of esterases in reprodutive, digestive and fat-body tissue of adult cotton boll weevil, Anthonomus grandis. Biochem. Genet. 24:499–508.
Kambysellis, M. P., Johnson, F. M., and Richardson, R. H. (1968). Isozyme variability in species of the genus Drosophila. IV: Distribution of the esterases in the body tissues of D. aldrichiand D. mulleri. Biochem. Genet. 1:249–265.
Kapin, M. A., and Ahmad, S. (1980). Esterases in larval tissues of gypsymoth Lymantria dispar(L): Optimun assay conditions, quantification and characterization. Insect Biochem. 10:331–337.
Karunaratne, S. H. P. P., and Hemingway, J. (1996). Different insecticides select multiple carboxylesterase isozymes and different resistance levels from a single population of Culex quinquefasciatus. Pestic. Biochem. Physiol. 54:4–11.
Lapenta, A. S., Bicudo, H. E. M. C., Ceron, C. R., and Cordeiro, J. A. (1995). Esterase patterns of species in the Drosophila buzzatticluster. Cytobios 84:13–29.
Lapenta, A. S., Bicudo, H. E. M. C., Ceron, C. R., and Cordeiro, J. A. (1998). Esterase patterns and phylogenetic relationships of species in the Drosophila buzzatiicluster. Cytobios 96:95–107.
Lima-Catelani, A. R. A., and Bicudo, H. E. M. C. (1994). Chromosome studies in two Brazilian populations of Aedes aegypti. Cytobios 79:241–251.
Mane, S. D., Tompkins, L., and Richmond, R. C. (1983). Male esterase 6 catalyzes the synthesis of a sex pheromone in Drosophila melanogasterfemale. Science 222:419–421.
Mani, G. S., Cook, L. M., and Marvdashti, R. (1986). What can be learn about selection from gene frequency distribution? Genetics 114:971–982.
Miller, S., and Novak, R. (1983). A comparative study of esterases in two strains of Anopheline mosquitoes by isoelectric focusing. Int. J. Biochem. 15:1409–1415.
Motoyama, M., and Dauterman, C. (1974). The role of non-oxidative metabolism in organophosphorous resistance. J. Agric. Food Chem. 22:350–355.
Mourya, D. T., Hemingway, J., and Leake, C. J. (1993). Changes in enzyme titres with age in four geographical strains of Aedes aegyptiand their association with insecticide resistance. Med. Vet. Entomol. 7:11–16.
Mutero, A., Pralavorio, M., Bride, J. M., and Fournier,D. (1994). Resistance associated point mutations in insecticide-insensitive acetylcholinesterase. Proc. Nat. Acad. Sci. U.S.A. 91:5922–5926.
Nascimento, A. P., and Bicudo, H. E. M. de C. (2002). Esterase patterns and phylogenetic relationships of Drosophilaspecies in the saltanssubgroup (saltans group). Genetica (The Netherlands) 114:41–51.
Oakeshott, J. G., Van Papenrecht, E. A., Boyce, T. M., Healy, M. J., and Russel, R. J. (1993). Evolutionary genetics of Drosophilaesterases. Genetica 90:239–268.
Pasteur, N., Nancé, E., and Bons, N. (2001). Tissue localization of overproduced esterases in the mosquito Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 38:791–801.
Perez-Mendoza, J., Fabrick, J. A., Zhu, K. Y., and Baker, J. E. (2000). Alterations in esterases are associated with malathion resistance in Habrobracon hebetor (Hymenoptera: Braconidae). J. Econ. Entomol. 93:31–37.
Pralavorio, M., and Fournier,D. (1992). Drosophilaacethylcholinesterase: Characterization of different mutants resistant to insecticides. Biochem. Genet. 30:77–83.
Richmond, R. C., Gilbert, D. G., Sheehan, K. B., Gromko, M. H., and Butterworth, F. M. (1980). Esterase 6 and reproduction in Drosophila melanogaster. Science 207:1483–1485.
Saul, S. H., Guptavanij, P., and Craig, J. R. G. B. (1976). Genetic variability at an esterase locus in Aedes aegypti. Ann. Entomol. Soc. Am. 69:73–79.
Sekar, V., and Hageman, J. H. (1979). Specificity of the serine protease inhibitor, phenylmethylsulfonyl fluoride. Biochem. Biophys. Res. Commun. 89:474–478.
Sergeev, P. V., Panin, V. M., Pavlova, G. V., Kopantseva, M. R., Shostak, N. G., Bashkirov, V. N., Georgiev, G. P., and Korochkin, L. I. (1995). The expression of esterase S gene of Drosophila virilisin Drosophila melanogaster. FEBS Lett. 360:194–196.
Shanmugavelu, M., Baytan, A. R., Chesnut, J. D., and Bonning, B. C. (2000). A novel protein that binds juvenile hormone esterase in fat body tissue and pericardial cells of the tobacco hornworm Manduca sextaL. J. Biol. Chem. 275:1802–1806.
Siegfried, B. D., and Ono, M. (1993). Mechanisms of parathion resistance in the greenbug Schizaphis graminum (Rondani). Pestic. Biochem. Physiol. 45:24–33.
Steiner, W.W. M., and Johnson, W. E. (1973). Techniques for electrophoresis of Hawaian Drosophila. US-IBP. Island Ecosyst. Tech. Rep. 30:1–21.
Townson, H. (1972). Esterase polymorphism in Aedes aegypti:The genetics and Km values of electrophoretically heterogeneous forms. Ann. Tropic. Med. Parasitol. 66:255–266.
Trebatoski, A. M., and Craig, G. B. (1969). Genetics of an esterase in Aedes aegypti. Biochem. Genet. 3:383–392.
Vaughan, A., Rocheleau, T., and Ffrench-Constant, R. (1997). Site-directed mutagenesis of an acethylcholinesterase gene from the yellowfever mosquito Aedes aegypticonfers insecticide insensitivity. Exp. Parasitol. 87:237–244.
Wagner, U. G., Petersen, E. I., Scwab, H., and Kratky, C. (2002). Est B from Burkholderia gladioli, a novel esterase with a lactamase fold reveals steric factors to discriminate between esterolytic and lactam cleaving activity. Protein Sci. 11:467–478.
Zhu, K. Y., and Gao, J. R. (1999). Increased activity associated with reduced sensitivity of acetylcholinesterase organophosphate-resistant greenbug, Schizaphis graminum (homoptera: Aphidae). Pestic. Sci. 55:11–17.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Abreu Lima-Catelani, A.R., Ceron, C.R. & de Campos Bicudo, H.E.M. Variation of Genetic Expression During Development, Revealed by Esterase Patterns in Aedes aegypti (Diptera, Culicidae). Biochem Genet 42, 69–84 (2004). https://doi.org/10.1023/B:BIGI.0000020463.89675.f0
Issue Date:
DOI: https://doi.org/10.1023/B:BIGI.0000020463.89675.f0