Abstract
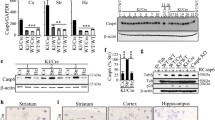
Caspase-3 is a potential therapeutic target for a number of degenerative diseases. However the development of specific caspase-3 inhibitors has been hampered by inter-species differences and the high degree of homology shared by different caspases. To circumvent these issues, we have produced and characterised a humanised caspase-3 mouse line (possessing one copy of the human gene with both copies of the murine gene disrupted) by crossing human caspase-3 transgenic mice with nullizygous caspase-3 knock-out mice. Humanised mice appeared normal and survived to adulthood. Analysis of the human gene revealed that human pro-caspase-3 was expressed in the same tissues as its murine counterpart. However humanised mice retained the hypercellularity of frontal cortex seen in their knock-out parental line and there was no biochemical evidence of human protein processing during naturally occurring neuronal death taking place during brain development. In contrast, the human protein was cleaved by the mouse machinery following anti-Fas treatment of adult mice. These data suggest that there is a fundamental difference between the activation pathways leading to caspase-3 cleavage during naturally occurring cell death in development/embryogenesis and following an apoptotic stimulus in the adult.
Similar content being viewed by others
References
Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 1999; 6: 1028–1042.
Slee EA, Adrain C, Martin SJ. Executioner caspases-3,-6 and-7 perform distinct, non-redundant, roles during the demolition phase of apoptosis. J Biol Chem 2001; 276: 7320–7326.
Hara H, Friedlander RM, Gagliardini V, et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA 1997; 94: 2007–2012.
Endres M, Namura S, Shimizu-Sasamata M, et al. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab 1998; 18: 238–247.
Shimohama S, Tanino H, Fujimoto S. Changes in caspase expression in Alzheimer's disease: Comparision with development and aging. Biochem Biophys Res Commun 1999; 256: 381–384.
Stadelmann C, Deckwerth TL, Srinivasan A, et al. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease. Evidence for apoptotic cell death. Am J Pathol 1999; 155: 1459–1466.
Masumura M, Hata R, Nishimura I, Uetsuki T, Sawada T, Yoshikawa K. Caspase-3 activation and inflammatory responses in rat hippocampus inoculated with a recombinant adenovirus expressing the Alzheimer amyloid precursor protein. Mol Brain Res 2000; 80: 219–227.
Miyashita T, Matsui J, Ohtsuka Y, et al. Expression of extended polyglutamine sequentially activates initiator and effector caspases. Biochem Biophys Res Commun 1999; 257: 724–730.
Sanchez-Mejia RO, Friedlander RM. Caspases in Huntington's disease. Neuroscientist 2001; 7: 480–489.
Yakovlev AG, Knoblach SM, Fan L, Fox GB, Goodnight R, Faden AI. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J Neurosci 1997; 17: 7415–7424.
Beer R, Franz G, Srinivasan A, et al. Temporal profile and cell subtype distribution of activated caspase-3 following experimental brain injury. J Neurochem 2000; 75: 1264–1273.
Knoblach SM, Nikolaeva M, Huang X, et al. Multiple caspases are activated after traumatic brain injury: Evidence for involvement in functional outcome. J Neurotrauma 2002; 19: 1155–1170.
Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol 1999; 45: 421–429.
Robertson GS, Crocker SJ, Nicholson DW, Schulz JB. Neuroprotection by the inhibition of apoptosis. Brain Pathol 2000; 10: 283–292.
Nuttall ME, Lee D, McLaughlin BA, Erhardt JA. Selective inhibitors of apoptotic caspases: Implications for novel therapeutic strategies. Drug Discov Today 2001; 6: 85–91.
Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Ann Rev Biochem 1999; 68: 383–424.
Boyce M, Degterev A, Yuan J. Caspases: An ancient cellular sword of Damocles. Cell Death Differ 2004; 11: 29–37.
Wheeler DL, Church DM, Federhen S, et al. Database resources of the National Center for Biotechnology. Nuc Acids Res 2003; 31: 28–33.
Fujita E, Jinbo A, Matuzaki H, Konishi H, Kikkawa U, Momoi T. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem Biophys Res Commun 1999; 264: 550–555.
Saleh M, Vaillancourt JP, Graham RK, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 2004; 429: 75–79.
Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000; 403: 98–103.
Ussat S, Werner UE, Adam-Klages S. Species-specific differences in the usage of several caspase substrates. Biochem Biophys Res Commun 2002; 297: 1186–1190.
Juan TSC, McNiece IK, Argento JM, et al. Identification and mapping of Casp7, a cysteine protease resembling CPP32, interleukin-1 converting enzyme, and CED-3. Genomics 1997; 40: 86–93.
Lippke JA, Gu Y, Sarnecki C, Caron PR, Su MSS. Identification and characterization of CPP32/Mch2 homolog 1, a novel cysteine protease similar to CPP32. J Biol Chem 1996; 271: 1825–1828.
Denault JB, Salvesen GS. Human caspse-7 activity and regulation by its N-terminal peptide. J Biol Chem 2003; 278: 34042–34050.
Srinivisan A, Roth KA, Sayers RO, et al. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ 1998; 5: 1004–1016.
Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 1998; 273: 32608–32613.
Schotte P, Declercq W, van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett 1999; 442: 117–121.
Lee D, Long SA, Murray JH, et al. Potent and selective non-peptide inhibitors of caspases 3 and 7. J Med Chem 2001; 44: 2015–2026.
Kerr LE, McGregor AL, Amet LEA, et al. Mice overexpressing human caspase 3 appear phenotypically normal but exhibit increased apoptosis and larger lesion volumes in response to transient focal cerebral ischaemia. Cell Death Differ 2004; 11: 1102–1111.
Cregan SP, MacLaurin JG, Craig CG, et al. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci 1999; 19: 7860–7869.
Keramaris E, Stephanis L, MacLauren J, et al. Involvement of caspase 3 in apoptotic death of cortical neurons evoked by DNA damage. Molec. Cell Neurosci 2000; 15: 368–379.
Kuida K, Zheng TS, Na S, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 1996; 384: 368–372.
Woo M, Hakem R, Soengas MS, et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev 1998; 12: 806–819.
Leonard JR, Klocke BJ, D'Sa C, Flavell RA, Roth KA. Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J. Neuropathol. Exp Neurol 2002; 61: 673–677.
Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual, 3rd ed. New York: Cold Spring Harbor Laboratory Press 2001.
Zheng TS, Hunot S, Kuida K, et al. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nature Med 2000; 6: 1241–1247.
Le DA, Wu Y, Huang Z, et al. Caspase activation and neuroprotection in caspase-3-deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci USA 2002; 99: 15188–15193.
Juan TSC, McNiece IK, Jenkins NA, Gilbert DJ, Copeland NG, Fletcher FA. Molecular characterization of mouse and rat CPP32 gene encoding a cysteine protease resembling interleukin-1 converting enzyme and CED-3. Oncogene 1996; 13: 749–755.
Pompeiano M, Blaschke AJ, Flavell RA, Srinivasan A, Chun J. Decreased apoptosis in proliferative and postmitotic regions of the caspase 3-deficient embryonic central nervous system. J Comp Neurol 2000; 423: 1–12.
Burek MJ, Oppenheim RW. Programmed cell death in the developing nervous system. Brain Pathol 1996; 6: 427–446.
Kuida K, Haydar TF, Kuan CY, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 1998; 94: 325–337.
Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci 2000; 23: 291–297.
de Bilbao F, Guarin E, Nef P, Vallet P, Giannakopoulos P, Dubois-Dauphin M. Postnatal distribution of cpp32/caspase 3 mRNA in the mouse central nervous system: An in situ hybridization study. J Comp Neurol 1999; 409: 399–357.
Momoi T, Fujita E, Urase K. Strain-specific caspase-3-dependent programmed cell death in the early developing mouse forebrain. Neuroreport 2003; 14: 111–115.
Urase K, Kouroku Y, Fujita E, Momoi T. Region of caspase-3 activation and programmed cell death in the early development of the mouse forebrain. Dev Brain Res 2003; 145: 241–248.
Roth KA, Kuan CY, Haydar TF, et al. Epistatic and independent functions of Caspase-3 and Bcl-XL in developmental programmed cell death. Proc Natl Acad Sci USA 2000; 97: 466–471.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kerr, L.E., Birse-Archbold, JL.A., Simon, A. et al. Differential regulation of caspase-3 by pharmacological and developmental stimuli as demonstrated using humanised caspase-3 mice. Apoptosis 9, 739–747 (2004). https://doi.org/10.1023/B:APPT.0000045787.50848.e1
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000045787.50848.e1