Abstract
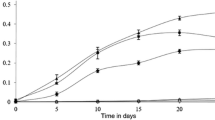
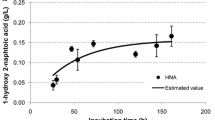
A bacterial strain ZL5, capable of growing on phenanthrene as a sole carbon and energy source but not naphthalene, was isolated by selective enrichment from crude-oil-contaminated soil of Liaohe Oil Field in China. The isolate was identified as a Sphingomonas sp. strain on the basis of 16S ribosomal DNA analysis. Strain ZL5 grown on phenanthrene exhibited catechol 2,3-dioxygenase (C23O) activity but no catechol 1,2-dioxygenase, gentisate 1,2-dioxygenase, protocatechuate 3,4-dioxygenase and protocatechuate 4,5-dioxygenase activities. This suggests that the mode of cleavage of phenanthrene by strain ZL5 could be meta via the intermediate catechol, which is different from the protocatechuate way of other two bacteria, Alcaligenes faecelis AFK2 and Nocardioides sp. strain KP7, also capable of growing on phenanthrene but not naphthalene. A resident plasmid (approximately 60 kb in size), designated as pZL, was detected from strain ZL5. Curing the plasmid with mitomycin C and transferring the plasmid to E. coli revealed that pZL was responsible for polycyclic aromatic hydrocarbons degradation. The C23O gene located on plasmid pZL was cloned and overexpressed in E. coli JM109(DE3). The ring-fission activity of the purified C23O from the recombinant E. coli on dihydroxylated aromatics was in order of catechol > 4-methylcatechol > 3-methylcatechol > 4-chlorocatechol ≫ 3,4-dihydroxyphenanthrene > 3-chlorocatechol.
Similar content being viewed by others
References
Bezalel L, Hadar Y, Fu PP, Freeman JP & Cerniglia CE (1996) Metabolism of phenanthrene by the White Rot Fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 62: 2547–2553
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Cain RB (1966) Utilization of anthranilic and nitrobenzoic acids by Nocardia opaca & Flavobacterium. J. Gen. Micro-biol. 42: 219–235
Chakrabarty AM (1972) Genetic basis of the biodegradation of salicylate in Pseudomonas. J. Bacteriol. 112: 815–823
Cho JC & Kim SJ (2001) Detection of mega plasmid from polycyclic aromatic hydrocarbon-degrading Sphingomonas sp. strain KS14. J. Mol. Microbiol. Biotechnol. 3: 503–506
Crawford RL, Button SW & Chapman PJ (1975) Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J. Bacteriol. 121: 794–799
Dunn N & Gunsalus IC (1973) Transmissible plasmids coding early enzyme of naphthalene oxidation in Pseudomonas putida. J. Bacteriol. 114: 974–979
Fuenmayor SL, Wild M, Boyes AL & Williams PA (1998) A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J. Bacteriol. 180: 2522–2530
Grimm AC & Harwood CS (1999) NahY, a catabolic plasmid-encoded receptor required for chemotaxis of Pseudomonas putida to the aromatic. J. Bacteriol. 181: 3310–3316
Johnsen AR, Winding A, Karlson U & Roslev P (2002) Linking of microorganisms to phenanthrene metabolism in soil by analysis of (13)C-labeled cell lipids. Appl. Environ. Micro-biol. 68: 6106–6113
Kado CI & Liu ST (1981) Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145: 1365–1373
Kanaly RA & Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bac-teria. J. Bacteriol. 182: 2059–2067
Kang BS, Ha JY, Lim JC, Lee J, Kim CK, Min KR & Kim Y (1998) Structure of catechol 2,3-dioxygenase gene from Alcaligenes eutrophus 335. Biochem. Biophys. Res. Commun. 245: 791–796
Kastner M & Mahro B (1996) Microbial degradation of polycyclic aromatic hydrocarbons in soil affected by the organic matrix of compost. Appl. Microbiol. Biotechnol. 44: 668–675
Kiyohara H, Nagao K & Kouno K (1982a) Phenanthrene-degrading phenotype of Alcaligenes faecelis AFK2. Appl. Environ. Microbiol. 43: 458–461
Kiyohara H, Nagao K & Yano K (1982b) Rapid screening for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl. Environ. Microbiol. 43: 454–457
Kojima Y, Itada N & Hayaishi O (1961) Metapyrocatechase: a new catechol-cleaving enzyme. J. Biol. Chem. 236: 2223–2228
LaVoce EI, Hecht SS, Bedenko V & Hoffman D (1982) Identification of the mutagenic metabolites of fluorene, 2-methylfluorene, and 3-methyl-fluoranthene. Carcinogenesis 3: 841–846
Lotufo GR (1997) Toxicity of sediment-associated PAHs to an estuarine copepod: effects on survival, feeding, reproduction and behaviour. Mar. Environ. Res. 44: 149–166
Meyer S, Moser R, Neef A, Stahl U & Kampfer P (1999) Differential detection of key enzymes of polyaromatic-hydrocarbon-degrading bacteria using PCR and gene probes. Microbiol. 145: 1731–1741
Romine MF, Stillwell LC, Wong KK, Thurston SJ, Sisk EC, Sensen C, Gaasterland T, Fredrickson JK & Saffer JD (1999) Complete sequence of a 184-kilobase catabolic plasmid from Sphingomonas aromaticivorans F199. J. Bacteriol. 181: 1585–1602
Saito A, Iwabuchi T & Harayama S (2000) A novel phenan-threne dioxygenase from Nocardioides sp. strain KP7: expression in Escherichia coli. J. Bacteriol. 182: 2134–2141
Sambrook J, Fritsch EF & Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanseverino J, Applegate BM, Henry King JM & Sayler GS (1993) Plasmid-mediated mineralization of naphthalene, phenanthrene, and anthracene. Appl. Environ. Microbiol. 59: 1931–1937
Stanier RY & Ingraham JL (1954) Protocatechuic acid oxidase. J. Biol. Chem. 210: 799–808
Takizawa N, Kaida N, Torigoe S, Moritani T, Sawada T, Satoh S & Kiyohara H (1994) Identification and characterization of genes encoding polycyclic aromatic hydrocarbon dioxygenase and polycyclic aromatic hydrocarbon dihydrodiol dehydro-genase in Pseudomonas putida OUS82. J. Bacteriol. 176: 2444–2449
Weisburg WG, Barns SM, Pelletier DA & Lane DJ (1991) 16s ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173: 697–703
Wheelis ML, Palleroni NJ & Stanier RY (1967) The metabolism of aromatic acids by Pseudomonas testeroni and P. acidivorans. Arch. Mikrobiol. 59: 302–314
Yrjala K, Suomalainen S & Suhonen EL (1998) Characterization and reclassification of an aromatic- and chloroaromatic-degrading Pseudomonas sp., sttain HV3, as Sphingomonas sp. HV3. Int J. Syst. Bacteriol. 48: 1057–1062
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, J. & Zhang, Z. Isolation and characterization of polycyclic aromatic hydrocarbons-degrading Sphingomonas sp. strain ZL5. Biodegradation 15, 205–212 (2004). https://doi.org/10.1023/B:BIOD.0000026579.38741.e1
Issue Date:
DOI: https://doi.org/10.1023/B:BIOD.0000026579.38741.e1