Abstract
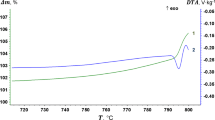
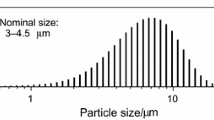
The kinetics of oxidation of copper powders in oxygen and in dry and humid air was investigated using thermogravimetric analysis (TGA). The extent of oxidation grew linearly with time until the weight-based thickness of the oxide film reached 0.13–1.22 nm, depending on the temperature. Between 30 and 90°C there was little difference between the kinetic curves observed in air and in oxygen, respectively. Higher humidity of the air resulted in an increased oxidation rate. Following the initial linear segment, the oxidation kinetics could be best described in terms of a logarithmic rate law between 30 and 45°C and in terms of a power law between 60 and 90°C. The activation energy for the initial linear stage was (44±2) kJ and for the subsequent oxidation (102±12) kJ. Delayed increases in oxidation rate were observed with a ca. 0.1-μm powder around 100°C, with a ca. 1-μm powder around 320°C, and with a < 10μm powder around 360°C. A three-stage model consisting of an initial linear stage, parabolic growth culminating in cracking of the oxide film, and subsequent re-start of the parabolic growth, gave good agreement with the experimental data. Whenever the powder is relatively uniform and the distribution of film-cracking times among the powder grains is narrow, e.g., within 23% of the median cracking time, an increase in the oxidation rate of the entire sample can be observed.
Similar content being viewed by others
REFERENCES
C. Wagner and K. Grunewald, Z. physikal. Chem. B 40, 455(1938).
R. F. Tylecote, J. Inst. Met. 78, 259(1950/1951).
A. Ronnquist and H. Fischmeister, J. Inst. Met. 89, 65(1960/1961).
S. Mrowec and A. Stoklosa, Bull. Acad. Polon. Sci. 18, 531(1970).
S. K. Roy, S. K. Bose, and S. C. Sircar, Oxid. Met. 35, 1(1991).
T. N. Rhodin, Jr., J. Am. Chem. Soc. 72, 5102(1950).
S. K. Roy and S. C. Sircar, Oxid. Met. 15, 9(1981).
R. F. Tylecote, J. Inst. Met. 81, 681(1952/1953).
R. F. Tylecote, Metallurgia 53, 191(1956).
D. W. Bridges, J. P. Baur, G. S. Baur, and W. M. Fassell, J. Electrochem. Soc. 103, 475(1957).
R. Haugsrud, J. Electrochem. Soc. 149, B14(2002).
H. Uhlig and R. Revie, Corrosion and Corrosion Control, (Wiley, New York, NY, 1985) p. 201.
Y. Z. Hu, R. Sharangpani, and S.-P. Tay, J. Electrochem. Soc. 148, G669(2001).
R. Guan, H. Hashimoto, and T. Yoshida, Acta Cryst. B40, 109(1984).
M. Lenglet and K. Kartouni, Rev. Metall. 90, 1638(1993).
A. M. Khoviv, I. N. Nazarenko, and A. A. Churikov, Inorg. Mat. 37, 473(2001).
N. B. Pilling and R. E. Bedworth, J. Inst. Met. 29, 529(1923).
N. Cabrera and N. F. Mott, Rept. Prog. Phys. 12, 263(1948–1949).
A. Yanase, H. Matsui, K. Tanaka, and H. Komiyama, Surf. Sci. 219, L601(1989).
P. Pascal, Nouveau Traite de Chimie Minerale, Vol. 1 (Masson et Cie., Paris, 1956) p. 633.
W. H. J. Vernon, J. Chem. Soc., 2273(1926).
R. J. Nika and P. M. Hall, IEEE Trans. Compon. Hybrids Manuf. Technol. CHMT-2, 412(1979).
S. Nakayama, A. Kimura, M. Shibata, S. Kuwabata, and T. Osakai, J. Electrochem. Soc. 148, B467(2001).
M. Hamalainen and I. Iivari, Suom. Kemistil. B 41, 37(1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feng, Z., Marks, C.R. & Barkatt, A. Oxidation-Rate Excursions During the Oxidation of Copper in Gaseous Environments at Moderate Temperatures. Oxidation of Metals 60, 393–408 (2003). https://doi.org/10.1023/A:1027331605417
Issue Date:
DOI: https://doi.org/10.1023/A:1027331605417