Abstract
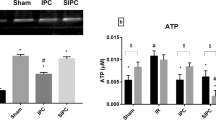
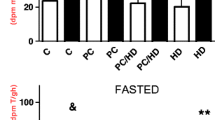
A short period of ischemia followed by reperfusion (ischemic preconditioning) is known to trigger mechanisms that contribute to the prevention of ATP depletion. In ischemic conditions, most of the ATP hydrolysis can be attributed to mitochondrial F1F0-ATPase (ATP synthase). The purpose of the present study was to examine the effect of myocardial ischemic preconditioning on the kinetics of ATP hydrolysis by F1F0-ATPase. Preconditioning was accomplished by three 3-min periods of global ischemia separated by 3 min of reperfusion. Steady state ATP hydrolysis rates in both control and preconditioned mitochondria were not significantly different. This suggests that a large influence of the enzyme on the preconditioning mechanism may be excluded. However, the time required by the reaction to reach the steady state rate was increased in the preconditioned group before sustained ischemia, and it was even more enhanced in the first 5 min of reperfusion (101 ± 3.0 sec in preconditioned vs. 83.4 ± 4.4 sec in controls, p ≶ 0.05). These results suggest that this transient increase in activation time may contribute to the cardioprotection by slowing the ATP depletion in the very critical early phase of post-ischemic reperfusion.
Similar content being viewed by others
References
Piper HM, Noll T, Siegmund B: Mitochondrial function in the oxygen depleted and reoxygenated myocardial cell. Cardiovas Res 28: 1–15, 1994
Harris DA, Das AM: Control of mitochondrial ATP synthesis in the heart. Biochem J 280: 561–573, 1991
Rouslin W, Erickson JLE, Solaro RJ: Effects of oligomycin and acidosis on rates of ATP depletion in ischemic heart muscle. Am J Physiol 250: H503–H508, 1986
Jennings RB, Reimer KA, Steenbergen C Jr: Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J Mol Cell Cardiol 23: 1383–1395, 1991
Murry CE, Jennings RB, Reimer KA: Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986
Walker DM, Yellon DM: Ischemic preconditioning: From mechanisms to exploitation. Cardiovasc Res 26: 734–739, 1992
Garnier A, Rossi A, Lavanchy N: Importance of the early alterations of energy metabolism in the induction and disappearance of ischemic preconditioning in the isolated rat heart. J Mol Cell Cardiol 28: 1671–1682, 1996
De Jong JW, De Jonge R, Marchesani A, Janssen M, Bradamante S: Controversies in preconditioning. Cardiovasc Drugs Ther 10: 767–773, 1997
Vuorinen K, Ylitalo K, Peuhkurinen K, Raatikainen P, Alarami A, Hassinen IE: Mechanism of ischemic preconditioning in rat myocardium. Roles of adenosine, cellular energy state, and mitochondrial F1F0-ATPase. Circulation 91: 2810–2818, 1995
Green DW, Murray HN, Sleph PG, Wang FL, Baird AJ, Rogers WL, Grover GJ: Preconditioning in rat hearts is independent of mitochondrial F1F0 ATPase inhibition. Am J Physiol 274: H90–H97, 1998
Das AM, Harris DA: Reversible modulation of the mitochondrial ATP synthase with energy demand in cultured rat cardiomyocytes. FEBS Lett 256: 97–100, 1989
Murry CE, Richard VJ, Reimer KA, Jennings RB: Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during sustained ischemia. Circ Res 66: 913–931, 1990
Yabe KI, Nasa Y, Sato M, Iijima R, Takeo S: Preconditioning preserves mitochondrial function and glycolytic flux during an early period of reperfusion in perfused rat hearts. Cardiovasc Res 33: 677–685, 1997
Hassinen IE, Vuorinen KH, Ylitalo K, Alarami A: Role of cellular energetics in ischemia-reperfusion and ischemic preconditioning of myocardium. Mol Cell Biochem 184: 393–400, 1998
Kobara M, Tatsumi T, Matoba S, Yamahara Y, Nakagawa C, Ohta B, Liu Y, Downey JM: Ischemic preconditioning protects against infarction in rat heart. Am J Physiol 263: H1107–H1112, 1992
Vasilyeva EA, Minkov IB, Fitin A.F, Vinogradov AD: Kinetic mechanism of mitochondrial adenosine triphosphatase. Biochem J 202: 9–14, 1982
Rouslin W: Factors affecting the reactivation of the oligomycin-sensitive adenosine 5'-triphosphatase and the release of ATPase inhibitor protein during the re-energization of intact mitochondria from ischemic cardiac muscle. J Biol Chem 262: 3472–3476, 1987
Zucchi R, Ronca-Testoni S, Yu G, Galbani P, Ronca G, Mariani M: Effect of ischemia and reperfusion on cardiac ryanodine receptorssarcoplasmic reticulum Ca2+ channels. Circ Res 74: 271–280, 1994
Vivaldi MT, Kloner RA, Schoen FJ: Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol 121: 522–530, 1985
Liu Y, Downey JM: Ischemic preconditioning protects against infarction in rat heart. Am J Physiol 263: H1107–H1112, 1992
Bradford MM: A refined and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248, 1976
Solaini G, Baracca A, Parenti-Castelli G, Strambini GB: Tryptophan phosphorescence as a structural probe of mitochondrial F1-ATPase e-subunit. Eur J Biochem 214: 729–734, 1993
Glantz SA: Primer of biostatistics. New York: McGraw-Hill, 1997
Klein G, Satre M, Dianoux AC, Vignais PV: Photoaffinity labelling of mitochondrial adenosine triphosphatase by an azido derivative of the natural adenosine triphosphatase inhibitor. Biochemistry 20: 1339–1334, 1981
Schwerzmann K, Pedersen PL: Proton-adenosinetriphosphatase complex of rat liver mitochondria: Effect of energy state on its interaction with the adenosine triphosphate inhibitor peptide. Biochemistry 20: 6305–6311, 1981
Husain I, Jackson PJ, Harris DA: Interaction between F1-ATPase and its naturally occurring inhibitor protein. Studies using a specific anti-inhibitor antibody. Biochim Biophys Acta 767: 64–74, 1985
Zucchi R, Ronca-Testoni S, Yu G, Galbani P, Ronca G, Mariani M: Postischemic changes in cardiac sarcoplasmic reticulum Ca2+ channels. Circ Res 76: 1049–1056, 1995
Yasuda R, Noji H, Kinosita ? Jr, Yoshida M: F1-ATPase is a highly efficient molecular motor that rotates with discrete 120°steps. Cell 93: 1117–1124, 1998
Lopez-Mediavilla C, Vigny H, Godinot C: Docking the mitochondrial inhibitor protein IF1 to a membrane receptor different from the F1-ATPase β subunit. Eur J Biochem 215: 481–490, 1993
Rouslin W: Regulation of the mitochondrial ATPase in situ in cardiac muscle. Role of the inhibitor subunit. J Bioenerg Biomembr 23: 873–887, 1991
Solaini G, Baracca A, Gabellieri E, Lenaz G: Modification of the mitochondrial F1-ATPase ε subunit, enhancement of the ATPase activity of the IF1-F1 complex and IF1-binding dependence of the conformation of the ε subunit. Biochem J 327: 443–448, 1997
Vander Heide RS, Hill ML, Reimer KA, Jennings RB: Effect of reversible ischemia on the activity of the mitochondrial ATPase: Relationship to ischemic preconditioning. J Mol Cell Cardiol 28: 103–112, 1996
Syroeshkin AV, Vasilyeva EA, Vinogradov AD: ATP synthesis catalyzed by the mitochondrial F1F0-ATP synthase is not a reversal of its ATPase activity. FEBS Lett 366: 29–32, 1995
Martins OB, Salgado-Martins I, Grieco MAB, Gomez-Puyou A, Tuena de Gomez Puyou: Binding of adenine nucleotides to the F1-inhibitor protein complex of bovine heart submitochondrial particles. Biochemistry 31: 5784–5790, 1992
Rouslin W, Broge CW: Regulation of mitochondrial matrix pH and adenosine 5'-triphosphatase during ischemia in slow heart-rate hearts: Role of Pi/H+ symport. J Biol Chem 264: 15224–15229, 1989
Van Raaij MJ, Orriss GL, Montgomery MG, Runswick MJ, Fearnley IM, Skehel JM, Walker JE: The ATPase inhibitor protein from bovine heart mitochondria: The minimal inhibitory sequence. Biochemistry 49: 15618–25, 1996
Hashimoto T, Yamamoto Y, Yoshida Y, Tagawa K: Cleavage of bovine mitochondrial ATPase inhibitor with endopeptidases, and binding of the resulting peptides to the interface between the α-and β-subunits of F1-ATPase. J Biochem 117: 641–647, 1995
Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ: Breakdown and release of myofilament proteins during ischemia and ischemia/ reperfusion in rat hearts. Identification of degradation products and effects on the pCa-force relation. Circ Res 82: 261–271, 1998
Kukreja RC, Janin Y: Reperfusion injury: Basic concepts and protection strategies. J Thromb Thromolysis 4: 7–24, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bosetti, F., Yu, G., Zucchi, R. et al. Myocardial ischemic preconditioning and mitochondrial F1F0-ATPase activity. Mol Cell Biochem 215, 31–38 (2000). https://doi.org/10.1023/A:1026558922596
Issue Date:
DOI: https://doi.org/10.1023/A:1026558922596