Abstract
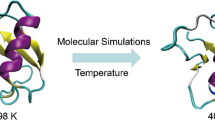
We have computed the average structures for the ras-p21 protein and its strongly homologous inhibitor protein, rap-1A, bound to the ras-binding domain (RBD) of the raf protein, using molecular dynamics. Our purpose is to determine the differences in structure between these complexes that would result in no mitogenic activity of rap-1A-RBD but full activity of p21-RBD. We find that despite the similarities of the starting structures for both complexes, the average structures differ considerably, indicating that these two proteins do not interact in the same way with this vital target protein. p21 does not undergo major changes in conformation when bound to the RBD, while rap-1 A undergoes significant changes in structure on binding to the RBD, especially in the critical region around residue 61. The p21 and rap-1A make substantially different contacts with the RBD. For example, the loop region from residues 55–71 of rap-la makes extensive hydrogen-bond contacts with the RBD, while the same residues of p21 do not. Comparison of the structures of the RBD in both complexes reveals that it undergoes considerable changes in structure when its structure bound to p21 is compared with that bound to rap-1A. These changes in structure are due to displacements of regular structure (e.g., α-helices and β-sheets) rather than to changes in the specific conformations of the segments themselves. Three regions of the RBD have been found to differ significantly from one another in the two complexes: the binding interface between the two proteins at residues 60 and 70, the region around residues 105–106, and 118–120. These regions may constitute effector domains of the RBD whose conformations determine whether or not mitogenic signal transduction will occur.
Similar content being viewed by others
REFERENCES
Adari, H., Lowy, D. R., Willumsen, B. F., Der, C. J., and McCormick, F. (1988). Science 240, 518–521.
Adler, V., Pincus, M. R., Brandt-Rauf, P. W., and Pincus, M. R. (1995). Proc. Natl. Acad. Sci. USA 92, 10585–10589.
Barbacid, M. (1987). Annu. Rev. Biochem. 56, 779–827.
Block, C., Janknecht, R., Herrmann, C., Nassar, N., and Wittinghofer, A. (1996). Nature Struct. Biol. 1996 (March), 244–258.
Bokoch, G. M. (1993). Biochem. J. 289, 17–24.
Chen, J., Lee, G., Murphy, R. B., Carty, R. P., Brandt-Rauf, P. W., Friedman, E., and Pincus, M. R. (1989). J. Biomol. Struct. Dynam. 6, 859–875.
Chen, J. M., Manolatos, S., Brandt-Rauf, P. W., and Pincus, M. R. (1996a). J. Protein Chem. 15, 11–16.
Chen, J. M., Grad, R., Monaco, R., Brandt-Rauf, P. W., and Pincus, M. R. (1996b). J. Protein Chem. 15, 11–16.
Chen, J. M., Brandt-Rauf, P. W., and Pincus, M. R. (1996c). J. Biomol. Struct. Dynam. 13, 925–933.
Clark, J., Drugan, J. K., Terrell, R. S., Bradham, C., Der, C. J., Bell, R. M., and Campbell, S. (1996). Proc. Natl. Acad. Sci. USA 93, 1577–1581.
Dykes, D. C., Brandt-Rauf, P. W., Luster, S. M., Friedman, F. K., and Pincus, M. R. (1993). J. Biomol. Struct. Dynam. 10, 905–918.
Emerson, S. D., Waugh, D. S., Scheffler, J. E., Tsao, K. L., Prinzo, K. M., and Fry, D. C. (1994). Biochemistry 33, 7745–7752.
Karin, M. (1995). J. Biol. Chem. 270, 16483–16486.
Leevers, S. J., Paterson, H. F., and Marshall, C. J. (1994). Nature 369, 411–414.
Liwo, A., Gibson, K. D., Scheraga, H. A., Brandt-Rauf, P. W., Monaco, R., and Pincus, M. R. (1994). J. Protein Chem. 13, 237–251.
Monaco, R., Chen, J. M., Chung, D. L., Brandt-Rauf, P. W., and Pincus, M. R. (1995a). J. Protein Chem. 14, 457–466.
Monaco, R., Chen, J. M., Friedman, F. K., Brandt-Rauf, P. W., and Pincus, M. R. (1995b). J. Protein Chem. 14, 721–730.
Moodie, S. A., Willumsen, B. M., Weber, M. J., and Wolfman, A. (1993). Science 260, 1658–1661.
Nassar, N., Horn, G., Herrmann, C., Scherer, A., McCormick, F., and Wittinghofer, A. (1995). Nature 375, 554–560.
Ne\(\begin{gathered} {\text{ }}\prime \hfill \\ {\text{m}} \hfill \\ \end{gathered} \)ethy, G., Pottle, M., and Scheraga, H. A. (1983). J. Phys. Chem. 87, 1883–1887.
Pai, E. F., Krengel, U., Petsko, G. A., Goody, R. S., Kabsch, W., and Wittinghofer, A. (1990). EMBO J. 9, 2351–2359.
Polakis, P., and McCormick, F. (1993). J. Biol. Chem. 268, 9157–9160.
Rodriguez-Viciana, P., Warne, P. H., Dhand, R., Vanhaesebroeck, B., Gout, I., Fry, M. J., Waterfield, M. D., and Downward, J. (1994). Nature 370, 527–532.
Smith, M. R., Liu, Y.-L., Kim, H., Rhee, S. G., and Kung, H.-F. (1990). Science 247, 1074–1077.
Vasquez, M., Nemethy, G., and Scheraga, H. A. (1983). Macromolecules 16, 1043–1049.
Weiner, S. J., Kollman, P. A., Case, D. A., Singh, V. C., Ghio, C., Alagona, G., Profeta, S., and Weiner, P. K. (1986). J. Am. Chem. Soc. 106, 765–784.
White, M. A., Nicolette, C., Minden, A., Polverino, A., von Aelst, L., Karin, M., and Wigler, M. (1995). Cell 80, 533–541.
Zhang, K., Noda, M., Vass, W. C., Papageorge, A. G., and Lowy, D. R. (1990). Science 249, 162–165.
Zhang, X.-F., Settleman, J., Kryiakis, J. M., Takeuchi-Suzuki, E., Elledge, S. J., Marshall, M. S., Bruder, J. T., Rapp, U. R., and Avruch, J. (1993). Nature 364, 308–313.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, J.M., Monaco, R., Manolatos, S. et al. Molecular Dynamics on Complexes of ras-p21 and Its Inhibitor Protein, rap-1A, Bound to the ras-Binding Domain of the raf-p74 Protein: Identification of Effector Domains in the raf Protein. J Protein Chem 16, 619–629 (1997). https://doi.org/10.1023/A:1026322924424
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1026322924424