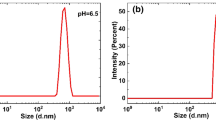
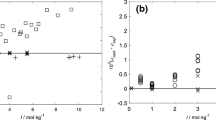
Abstract
Colloid forms of a series of microcomponents were studied in aqueous solutions by the colloidal chemical extraction method in combination with ICP MS. The potential of this combined method for determination of the total fraction of the colloid species and distribution of microcomponents of stable and radioactive elements throughout colloid forms at their natural abundance in potable water is examined.
Similar content being viewed by others
REFERENCES
Dupre, B., Viers, J., Dandurand, J.-L., et al., Chem. Geol., 1990, vol. 160, pp. 63¶80.
Gou, L. and Santschi, P.H., Marine Chem., 1997, vol. 59, pp. 1¶15.
Douglas, G.B., Hart, B.T., Beckett, R. et al., Aquatic Geochem., 1999, vol. 5, pp. 167
Buddenmeier, R.W. and Hunt, J.R., LLNL Preprint, 1988, no. UCRI-98 204.
Santschi, P., Gill, G., and Paternostro, Ch., Marine Chem., 1999, vol. 63, pp. 185¶212.
Muller, F.L.L., Anal. Chim. Acta, 1996, vol. 331, pp. 1¶15.
Wen, L.-S., Santschi, P.H., and Tang, D., Geochim. Cosmochim. Acta, 1997, vol. 61, no. 14, pp. 2867¶2892.
Lead, J.R., Hamilton-Taylor, J., Davison, W., and Harper M., Geochim. Cosmochim. Acta, 1999, vol. 63, nos. 11/12, pp. 1661¶1670.
Muller, F.L.L., Estuarine, Coastal Shelf Sci., 1998, vol. 46, pp. 419¶437.
Mounier, S., Braucher, R., and Benaxim, J.Y., Water Res., 1999, vol. 33, no. 10, pp. 2363¶2373.
Huisman, I.H. and Tragargh, Ch., Chem. Eng. Sci., 1999, vol. 54, pp. 271¶280.
Huisman, I.H. and Tragargh, Ch., Chem. Eng. Sci., 1999, vol. 54, pp. 281¶289.
Bellara, S.R. and Cui, Zh., Chem. Eng. Sci., 1998, vol. 53, no. 12, pp. 2153¶2166.
Carrére, H. and Rene, F., Exp. Fluids, 1998, vol. 25, pp. 243¶253.
Dai, M., Buesseler, K.O., Ripple, P., et al., Marine Chem., 1998, vol. 62, pp. 117¶136.
Ingri, J., Widerlund, A., Land, M., et al., Chem. Geol., 2000, vol. 166, pp. 23¶45.
Guo, L., Wen, L.-S., Degui, Tang, and Santschi, P.H., Marine Chem., 2000, vol. 69, pp. 75¶90.
Tombasz, E., Dobos, A.B., Szekeres, M., et al., Colloid Polym. Sci., 2000, vol. 278, pp. 337¶345.
Langford, C.H., in Coordination Chemistry, ACS, 1994, ch. 33, pp. 406¶417.
Jung, B., Edelstein, N.M., and Seaborg, G.T., in Coordination Chemistry. A Century of Progress, ACS, 1994, ch. 30, pp. 361¶379.
Pihong, Zhao and Steward, S.A., LLNL Preprint, 1997, no. UCRL-ID-126 039.
Wen, Liang-Saw, Santschi, P.H., and Defui, Tang, Geochim. Cosmochim. Acta, 1997, vol. 61, no. 14, pp. 2867¶2878.
Kretzshmar, R., Robarge, W.P., and Amoozegar, A., Water Resources Res., 1995, vol. 31, no. 3, pp. 435¶445.
Berkel, J. van and Beckett, R., J. Chromatogr., Ser. A, 1996, vol. 733, pp. 105¶117.
Beckett, R., At. Spectrosc., 1991, vol. 12, pp. 228¶232.
Beckett, R., Nicholson, G., Hotchin, D., and Hart, B., Hydrobiologia, 1992, vols. 235/236, pp. 697¶710.
Beckett, R., Nicholson G., Hart, B.T., et al., Water Res., 1988, vol. 22, pp. 1535¶1545.
Beckett, R., Bigelow, J.C., Jhang, J., and Giddings, J.C., Environ. Sci. Technol., 1988, vol. 21, pp. 289¶295.
Ran, Y., Fu, J.M., Sheng, G.Y., et al., Chemosphere, 2000, vol. 41, pp. 33¶43.
Ranville, J.F., Chittleborough, D.J., Shanks, F., et al., Anal. Chim. Acta, 1999, vol. 381, pp. 315¶329.
Douglas, G.B., Hart, B.T., Beckett, R., et al., Aquatic Geochem., 1999, vol. 5, pp. 167¶194.
Kersting, A.B., Efurd, D.W., Finnegan, D.L., et al., Nature, 1999, vol. 397, pp. 56¶59.
Beneš, P. and Majer, V., Trace Chemistry of Aqueous Solutions, Prague: Academia, 1980.
Laurence, E., Schemel, L.E., Briant, A., et al., Appl. Geochem., 2000, vol. 15, pp. 1003¶1018.
Polyakov, E.V., Sootnoshenie periodichnosti i monotonnosti v sisteme khimicheskikh elementov (Periodicity to Monotony Relationship in the System of Chemical Elements), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 1997.
Polyakov, E.V. and Egorov, Yu.V., Anal. Kontr., 2001, vol. 5, no. 3, pp. 219¶239.
Polyakov, E.V. and Egorov, Yu.V., Anal. Kontr., 2002, vol. 6, no. 1, pp. 3¶12.
Polyakov, E.V., Il'ves, G.N., and Surikov, V.T., Radiokhimiya, 2000, vol. 42, no. 5, pp. 427¶430.
Barysheva, N.M., Garmasheva, N.V., Polyakov, E.V., et al., Abstracts of Papers, XIII Int. Conf. “Chemistry for Protection of the Environment”, Hawaii, 2002, p. 11.
Sharaf, M.A., Illman, D.L., and Kowalski, B.R., Chemometrics, New York: Wiley, 1986.
Egorov, Yu.V., Statika sorbtsii mikrokomponentov oksigidratami (Statics of Sorption of Microcomponents on Hydroxides), Moscow: Atomizdat, 1975.
Davydov, Yu.P., Sostoyanie radionuklidov v rastvorakh (Radionuclide Speciation in Solutions), Minsk: Nauka i Tekhnika, 1978.
Polyakov, E.V., Il'ves, G.N., and Surikov, V.T., Radiokhimiya, 2000, vol. 42, no. 5, pp. 431¶434.
Polyakov, E.V., Egorov, Yu.V., and Il'ves, G.N., Czech. J. Phys., 1999, vol. 49, suppl. 1, pp. 773¶781.
Krainov, S.R. and Shvets, V.M., Osnovy geokhimii podzemnykh vod (Principles of Geochemistry of Groundwater), Moscow: Nedra, 1980.
Uhlig, H.H. and Revie, R.W., Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, New York: Wiley, 1985. Translated under the title Korroziya i bor'ba s nei, Leningrad: Khimiya, 1989, p. 106.
Starik, I.E., Osnovy radiokhimii (Principles of Radiochemistry), Leningrad, 1969.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Polyakov, E.V., Il'ves, G.N. & Surikov, V.T. Determination of Ionic to Colloid Form Ratio of Microcomponents in Fresh Water by Colloidal Chemical Extraction. Radiochemistry 45, 506–511 (2003). https://doi.org/10.1023/A:1026224312400
Issue Date:
DOI: https://doi.org/10.1023/A:1026224312400