Abstract
Purpose. The hepatic and intestinal metabolic activities of P450 were evaluated in rats with surgery- and drug-induced renal dysfunction.
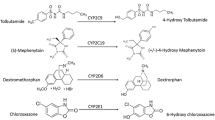
Methods. Renal failure was induced by five-sixths nephrectomy (NR), bilateral ureter ligation (BUL), the intramuscular injection of glycerol (GL), and the intraperitoneal injection of cisplatin (CDDP). Phenytoin 4-hydroxylation, debrisoquine 4-hydroxylation, and testosterone 6β-hydroxylation were estimated to evaluate the metabolic activities of cytochrome P450 (CYP) 2C, 2D, and 3A, respectively.
Results. The hepatic CYP3A metabolic activities were decreased by 65.9% and 60.2% in NR and GL rats, respectively. The hepatic CYP2C metabolic activity was decreased by 48.8% in CDDP rats. No alteration in hepatic drug-metabolizing activities was observed in BUL rats. On the other hand, the intestinal CYP3A metabolic activity was weakly increased in GL rats but not significantly altered in NR, CDDP, and BUL rats.
Conclusions. This study suggested (a) that only selected P450 metabolic activity in the liver is decreased in renal failure, (b) that extent of the decrease in hepatic metabolic activities of P450 is dependent on the etiology of renal failure, and (c) that alteration of CYP3A metabolic activity in the intestine is not always correlated with that in the liver.
Similar content being viewed by others
References
M. Touchette and R. Slaughter. The effect of renal failure on hepatic drug clearance. DICP 25:1214-1224 (1991).
S. Fukatsu, I. Yano, T. Igarashi, T. Hashida, K. Takayanagi, H. Saito, S. Uemoto, T. Kiuchi, K. Tanaka, and K. Inui. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur. J. Clin. Pharmacol. 57:479-484 (2001).
H. Yamazaki, T. Komatsu, K. Takemoto, M. Saeki, Y. Minami, Y. Kawaguchi, N. Shimada, M. Nakajima, and T. Yokoi. Decreased in phenytoin hydroxylation activities catalyzed by liver microsomal cytochrome P450 enzymes in phenytoin-treated rats. Drug Metab. Dispos. 29:427-434 (2001).
T. Schulz-Utermoehl, A. Bennett, A. Ellis, G. Tucker, A. Boobis, and R. Edwards. Polymorphic debrisoquine 4-hydroxylase activity in the rat is due to differences in CYP2D2 expression. Pharmacogenetics 9:357-366 (1999).
T. Johnson, M. Tanner, and G. Tucker. A comparison of the ontogeny of enterocytic and hepatic cytochrome P450 3A in the rat. Biochem. Pharmacol. 60:1601-1610 (2000).
H. Okabe, I. Yano, Y. Hashimoto, H. Saito, and K. Inui. Evaluation of increased bioavailability of tacrolimus in rats with experimental renal dysfunction. J. Pharm. Pharmacol. 54:65-70 (2002).
V. Lin, A. Dowling, S. Quigley, F. Farin, J. Zhang, J. Lamba, E. Schuetz, and K. Thummel. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol. Pharmacol. 62:162-172 (2002).
F. Leblond, M. Petrucci, P. Dube, G. Bernier, A. Bonnardeaux, and V. Pichette. Downregulation of intestinal cytochrome P450 in chronic renal failure. J. Am. Soc. Nephrol. 13:1579-1585 (2002).
L. Ji, S. Masuda, H. Saito, and K. Inui. Down-regulation of rat organic cation transporter rOCT2 by 5/6 nephrectomy. Kidney Int. 62:514-524 (2002).
M. Giacomini, M. Roberts, and G. Levy. Evaluation of methods for producing renal dysfunction in rats. J. Pharm. Sci. 70:117-121 (1981).
L. Shulman, Y. Yuhas, I. Frolkis, S. Gavendo, A. Knecht, and H. Eliahou. Glycerol induced ARF in rats is mediated by tumor necrosis factor-alpha. Kidney Int. 43:1397-1401 (1993).
H. Okabe, A. Mizukami, M. Taguchi, T. Aiba, M. Yasuhara, and Y. Hashimoto. Increased intestinal absorption rate is responsible for the reduced hepatic first-pass extraction of propranolol in rats with cisplatin-induced renal dysfunction. J. Pharm. Pharmacol. 55:479-486 (2003).
I. Mahmood and D. Waters. A comparative study of uranyl nitrate and cisplatin-induced renal failure in rats. Eur. J. Drug Metab. Pharmacokinet. 4:327-336 (1994).
H. Okabe, Y. Hashimoto, and K. Inui. Pharmacokinetics and bioavailability of tacrolimus in rats with experimental renal dysfunction. J. Pharm. Pharmacol. 52:1467-1472 (2000).
Y. Hashimoto, T. Aiba, M. Yasuhara, and R. Hori. Effect of experimental renal dysfunction on bioavailability of ajmaline in rats. J. Pharm. Pharmacol. 53:805-813 (2001).
T. Aiba, Y. Takehara, M. Okuno, and Y. Hashimoto. Poor correlation between intestinal and hepatic metabolic rates of CYP3A4 substrates in rats. Pharm. Res.
T. Komatsu, H. Yamazaki, S. Asahi, J. Gillam, P. Guengerich, M. Nakajima, and T. Yokoi. Formation of a dihydroxy metabolite of phenytoin by human liver microsomes/cytosol: Roles of cytochrome P450 2C9, 2C19, and 3A4. Drug Metab. Dispos. 28:1360-1368 (2000).
T. Kronbach. Bufuralol, dextromethorphan, and debrisoquine as prototype substrates for human P450IID6. Methods Enzymol. 206:509-517 (1991).
T. Ikeda, K. Nishimura, T. Taniguchi, T. Hata, E. Kashiyama, S. Kudo, G. Miyamoto, H. Kobayashi, S. Kobayashi, O. Okazaki, H. Hakusui, E. Aoyama, Y. Yoshimura, Y. Yamada, M. Yoshikawa, M. Otsuka, T. Niwa, A. Kagayama, S. Suzuki, and T. Sato. In vitro evaluation of drug interaction caused by enzyme inhibition. HAB protocol. Xenobio. Metab. Dispos. 16:115-126 (2001).
F. Leblond, C. Guevin, C. Demers, I. Pellerin, M. Barre, and V. Pichette. Downregulation of hepatic cytochrome P450 in chronic renal failure. J. Am. Soc. Nephrol. 12:326-332 (2001).
A. Mahnke, D. Strotkamp, P. Roos, W. Hanstein, G. Chaboot, and P. Nef. Expression and inducibility of cytochrome P450 3A9 (CYP3A9) and other members of the CYP3A subfamily in rat liver. Arch. Biochem. Biophys. 337:62-68 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okabe, H., Hasunuma, M. & Hashimoto, Y. The Hepatic and Intestinal Metabolic Activities of P450 in Rats with Surgery- and Drug-Induced Renal Dysfunction. Pharm Res 20, 1591–1594 (2003). https://doi.org/10.1023/A:1026131216669
Issue Date:
DOI: https://doi.org/10.1023/A:1026131216669