Abstract
Purpose
The aim of this study is to evaluate the relationship between the CYP450 enzyme family and cisplatin toxicity.
Methods
This article examined a collection of studies suggesting that CYP450 enzymes may influence cisplatin toxicity. We performed a narrative mini-review.
Results
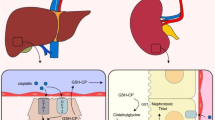
The studies review showed that CYP450 enzymes have an important role in drug-induced hepatotoxicity and nephrotoxicity, mainly CYP2E1 and CYP4A11. The studies also suggested that the cisplatin and CYP2E1 interaction leads to the generation of reactive oxygen species (ROS) and other oxidants resulting in renal injury; and that ROS generated by both the use of cisplatin and by the CYP2E1 increases tissue damage, induces apoptosis, and causes liver failure.
Conclusions
We observed that there is an important relationship between CYP450 and cisplatin, involving increased toxicity. However, the possible mechanisms described for the involvement of CYP450 enzymes in nephrotoxicity and hepatotoxicity induced by cisplatin need to be confirmed by further studies. Therefore, there is a need for a deeper investigation focusing on cisplatin toxicity mediated by CYP450 enzymes, which would undoubtedly contribute to a better understanding of the mechanisms that have been implicated so far.




Similar content being viewed by others
References
Lokich J, Anderson N (1998) Carboplatin versus cisplatin in solid tumors: an analysis of the literature. Ann Oncol 9:13–21
Gong P (2006) Modeling conformational dynamics of cisplatin and oxaliplatin adducts with DNA. Dissertation, University of North Carolina
O’Dwyer PJ, Stevenson JP, Johnson SW (2000) Clinical pharmacokinetics and administration of established platinum drugs. Drugs 59:19–27
Fuertes MA, Alonso C, Pérez JM (2003) Biochemical modulation of Cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev 103:645–662
Safaei R, Howell SB (2005) Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol 53:13–23
Ciarimboli G, Ludwig T, Lang D et al (2005) Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167:1477–1484
Klein AV, Hambley TW (2009) Platinum drug distribution in cancer cells and tumors. Chem Rev 109:4911–4920
Cepeda V, Fuertes MA, Castilla J et al (2007) Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 7:3–18
Johnson SW, Ferry KV, Hamilton TC (1998) Recent insights into platinum drug resistance in cancer. Drug Resist Updat 1:243–254
Fuertes MA, Castilla J, Alonso C et al (2003) Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 10:257–266
Ahmad S (2010) Platinum-DNA interactions and subsequent cellular processes controlling sensitivity to anticancer platinum complexes. Chem Biodivers 7:543–566
Chu E, DeVita VT (2008) Chemotherapeutic and biologic drugs. In: Chu E, McGowan M, Elfiky A et al (eds) Physician’s cancer chemotherapy drug manual 2008. Jones and Bartlett Publishers, Massachusetts, pp 94–98
Hall MD, Okabe M, Shen DW et al (2008) The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol 48:495–535
Hrubisko M, McGown AT, Fox BW (1993) The role of metallothionein, glutathione, glutathione S-transferases and DNA repair in resistance to platinum drugs in a series of L1210 cell lines made resistant to anticancer platinum agents. Biochem Pharmacol 7:253–256
Rabik CA, Dolan ME (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33:9–23
Nakayama K, Miyazaki K, Kanzaki A et al (2001) Expression and cisplatin sensitivity of copper-transporting P-type adenosine triphosphatase (ATP7B) in human solid carcinoma cell lines. Oncol Rep 8:1285–1287
Samimi G, Katano K, Holzer AK et al (2004) Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol 66:25–32
Blair BG, Larson CA, Safaei R et al (2009) Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin Cancer Res 15:4312–4321
Schrenk D, Baus PR, Ermel N et al (2001) Up-regulation of transporters of the MRP family by drugs and toxins. Toxicol Lett 120:51–57
Liedert B, Materna V, Schadendorf D et al (2003) Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G2-arrest in melanoma cells resistant to cisplatin. J Invest Dermatol 121:172–176
Jacobs C, Kalman SM, Tretton M et al (1980) Renal handling of cis-diamminedichloroplatinum(II). Cancer Treat Rep 64:1223–1226
Klasco RK (1974–2006) DRUGDEX system. Greenwood Village (Colorado): Thomson MICROMEDEX. http://www.periodicos.capes.gov.br. Accessed 16 Nov 2016
Go R, Adjel A (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 17:409–422
Belt RJ, Himmelstein KJ, Patton TF et al (1979) Pharmacokinetics of non-protein-bound platinum species following administration of cis-dichlorodiammineplatinum(II). Cancer Treat Rep 63:1515–1521
Kuhlmann MK, Burkhardt G, Kohler H (1997) Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant 12:2478–2480
Rosenberg B, Van Camp L, Trosko JE et al (1969) Platinum compounds: a new class of potent antitumour agents. Nature 222:385–386
Cvitkovic E (1998) Cumulative toxicities from cisplatin therapy and current cytoprotective measures. Cancer Treat Rev 24:265–281
Iraz M, Ozerol E, Gulec M et al (2006) Protective effect of caffeic acid phenethyl ester (CAPE) administration on cisplatin-induced oxidative damage to liver in rat. Cell Biochem Funct 24:357–361
Sahu BD, Kuncha M, Sindhura GJ et al (2013) Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 20:453–460
Santos NA, Catão CS, Martins NM et al (2007) Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol 81:495–504
Dehne N, Lautermann J, Petrat F et al (2001) Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol 174:27–34
Jiang Y, Guo C, Vasko MR et al (2008) Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res 68:6425–6434
Melli G, Taiana M, Camozzi F et al (2008) Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol 214:276–284
Santos NA, Bezerra CS, Martins NM et al (2008) Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother Pharmacol 61:145–155
Campbell KCM, Rybak LP, Meech RP et al (1996) D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res 102:90–98
Marullo R, Werner E, Degtyareva N et al (2013) Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 8:e81162
Masuda H, Tanaka T, Takahama U (1994) Cisplatin generates superoxide anion by interaction with DNA in a cell-free system. Biochem Biophys Res Commun 203:1175–1180
Tsutsumishita Y, Onda T, Okada K et al (1998) Involvement of H2O2 production in cisplatin-induced nephrotoxicity. Biochem Biophys Res Commun 242:310–312
Martins NM, Santos NA, Curti C et al (2008) Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol 28:337–344
Furukawa M, Nishimura M, Ogino D et al (2004) Cytochrome p450 gene expression levels in peripheral blood mononuclear cells in comparison with the liver. Cancer Sci 95:520–529
Song BJ, Veech RL, Saenger P (1990) Cytochrome P450IIE1 is elevated in lymphocytes from poorly controlled insulin-dependent diabetics. J Clin Endocrinol Metab 71:1036–1040
Meyer RP, Gehlhaus M, Knoth R et al (2007) Expression and function of cytochrome p450 in brain drug metabolism. Curr Drug Metab 8:297–306
Tanaka E (1998) Clinically important pharmacokinetic drug–drug interactions: role of cytochrome P450 enzymes. J Clin Pharm Ther 23:403–416
Schenkman JB, Gibson GG (1983) Status of the cytochrome P450 cycle. In: Lamble JW (ed) Drug metabolism and distribution. Elsevier Biomedical Press, Amsterdam, pp 7–11
Jaeschke H, Gores GJ, Cederbaum AI et al (2002) Mechanisms of hepatotoxicity. Toxicol Sci 65:166–176
Lieber CS (1997) Cytochrome P450 2E1: its physiological and pathological role. Physiol Rev 77:517–544
Hartman JH, Miller GP, Meyer JN (2017) Toxicological implications of mitochondrial localization of CYP2E1. Toxicol Res 6:273–289
Castillo T, Koop DR, Kamimura S et al (1992) Role of cytochrome P-450 2E1 in ethanol-, carbon tetrachloride and iron-dependent microsomal lipid peroxidation. Hepatology 16:992–996
Dai Y, Rashba-Step J, Cederbaum AI (1993) Stable expression of human cytochrome P4502E1 in HepG2 cells: characterization of catalytic activities and production of reactive oxygen intermediates. Biochemistry 32:6928–6937
Sakurai K, Cederbaum AI (1998) Oxidative stress and cytotoxicity induced by ferric-nitrilotriacetate in HepG2 cells that express cytochrome P450 2E1. Mol Pharmacol 54:1024–1035
Wang X, Lu Y, Cederbaum AI (2005) Induction of cytochrome P450 2E1 increases hepatotoxicity caused by Fas agonistic Jo2 antibody in mice. Hepatology 42:400–410
Lu Y, Wang X, Cederbaum AI (2005) Lipopolysaccharide-induced liver injury in rats treated with the CYP2E1 inducer pyrazole. Am J Physiol Gastrointest Liver Physiol 289:G308–G319
Pellinen P, Honkakoski P, Stenbäck F et al (1994) Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol 270:35–43
Cummings BS, Zangar RC, Novak RF et al (1999) Cellular distribution of cytochromes P-450 in the rat kidney. Drug Metab Dispos 27:542–548
Knights KM, Rowland A, Miners JO (2013) Renal drug metabolism in humans: the potential for drug–endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT). Br J Clin Pharmacol 76:587–602
Anders MW, Dekant W (1993) Renal xenobiotic metabolism: role in bioactivation of nephrotoxic xenobiotics. In: Anders MW, Dekant W, Henschler D et al (eds) Renal disposition and nephrotoxicity of xenobiotics. Academic, San Diego, pp 155–183
Ohkawa H, Hisada Y, Fujiwara N et al (1974) Metabolism of N-(3′,5′-dichlorophenyl) succinimide in rats and dogs. Agric Biol Chem 38:1359–1369
Griffin RJ, Rutt DB, Henesey CM et al (1996) In vitro metabolism of the nephrotoxicant A/-(3,5-dichlorophenyl) succinimide in the Fischer 344 rat and New Zealand white rabbit. Xenobiotica 26:369–380
Liu S, Yao Y, Lu S et al (2013) The role of renal proximal tubule P450 enzymes in chloroform-induced nephrotoxicity: utility of renal specific P450 reductase knockout mouse models. Toxicol Appl Pharmacol 272:230–237
Nyarko AK, Kellner-Weibel GL, Harvison PJ (1997) Cytochrome P450-mediated metabolism and nephrotoxicity of N-(3,5-dichlorophenyl) succinimide in Fischer 344 rats. Fundam Appl Toxicol 37:117–124
Smith JH, Hook JB (1984) Mechanism of chloroform nephrotoxicity. III. Renal and hepatic microsomal metabolism of chloroform in mice. Toxicol Appl Pharmacol 73:511–524
Constant AA, Sprankle CS, Peters JM et al (1999) Metabolism of chloroform by cytochrome P450 2E1 is required for induction of toxicity in the liver, kidney, and nose of male mice. Toxicol Appl Pharmacol 160:120–126
Mitchell JR, McMurtry RJ, Statham CN et al (1977) Molecular basis for several drug-induced nephropathies. Am J Med 62:518–526
Scripture CD, Sparreboom A, Figg WD (2005) Modulation of cytochrome P450 activity: implications for cancer therapy. Lancet Oncol 6:780–789
Li J, Li D, Tie C et al (2015) Cisplatin-mediated cytotoxicity through inducing CYP4A11 expression in human renal tubular epithelial cells. J Toxicol Sci 40:895–900
Masubuchi Y, Kawasaki M, Horie T (2006) Down-regulation of hepatic cytochrome P450 enzymes associated with cisplatin-induced acute renal failure in male rats. Arch Toxicol 80:347–353
Lu Y, Cederbaum AI (2006) Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci 89:515–523
Liu H, Baliga R (2003) Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int 63:1687–1696
Liu H, Baliga M, Baliga R (2002) Effect of cytochrome P450 2E1 inhibitors on cisplatin-induced cytotoxicity to renal proximal tubular epithelial cells. Anticancer Res 22:863–868
Baliga R, Zhang Z, Baliga M et al (1998) Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int 54:1562–1569
Caro AA, Cederbaum AI (2003) Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol 44:27–42
Czekaj P, Wiaderkiewiez A, Florek E et al (2005) Tobacco smoke-dependent changes in cytochrome P450 1A1, 1A2, and 2E1 protein expressions in fetuses, newborns, pregnant rats, and human placenta. Arch Toxicol 79:13–24
Ingelman-Sundberg M, Oscarson M, Daly AK et al (2001) Human cytochrome P-450 (CYP) genes a web page for the nomenclature of alleles. Cancer Epidemiol Biomarkers Prev 10:1307–1308
Hayashi S, Watanabe J, Kawajiri K (1991) Genetic polymorphisms in the 5′-flanking region change transcriptional regulation of the human cytochrome P450IIEI gene. J Biochem 110:559–565
Watanabe J, Hayashi S, Sawajiri K (1994) Different regulation and expression of the human CYP2E1 gene due to the RsaI polymorphism in the 5′-flanking region. J Biochem 116:321–326
Nomura F, Itoga S, Uchimoto T et al (2003) Transcriptional activity of the tandem repeat polymorphism in the 5′-flanking region of the human CYP2E1 gene. Alcohol Clin Exp Res 27:42S–46S
Uematsu F, Kikuchi H, Motomiya M et al (1991) Association between restriction fragment length polymorphism of the human cytochrome P450IIEI gene and susceptibility to lung cancer. Jpn J Cancer Res 82:254–256
Khrunin AV, Moisseev A, Gorbunova V et al (2010) Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J 10:54–61
Acknowledgements
We are thankful to the São Paulo Research Foundation (FAPESP) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author JCFQ declares that she has no conflict of interest. Author VMS declares that she has no conflict of interest. Author MBV declares that she has no conflict of interest. Author LSA declares that she has no conflict of interest. Author RMMS declares that she has no conflict of interest. Author TZ declares that he has no conflict of interest. Author LAS declares that he has no conflict of interest. Author PM declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Quintanilha, J.C.F., de Sousa, V.M., Visacri, M.B. et al. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol 80, 223–233 (2017). https://doi.org/10.1007/s00280-017-3358-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3358-x