Abstract
Purpose. To study mixtures of SDS and the drugs diphenhydramine, tetracaine, and amitriptyline to compile phase diagrams and to investigate the use of interesting phases for sustained release from gels.
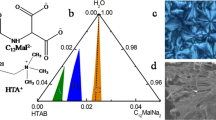
Methods. Phase diagrams were composed by studying large numbers of different compositions of negatively charged SDS and positively charged drug compounds visually, rheologically, and by cryo-transmission electron microscopy. Drug release from Carbopol 940 and agar gels containing interesting phases, e.g., vesicle and branched micelle phases, was measured in vitro by the USP paddle method.
Results. Vesicles and elongated and branched micelles were formed on the SDS-rich side in all three systems examined. The tetracaine system differed from the other two in that it showed a vesicle area in the drug-rich side. Release of diphenhydramine from Carbopol 940 gels was slowed by at least a factor of 10 when in the form of vesicles or branched micelles. The same delay was found for both drug-rich and SDS-rich tetracaine vesicles.
Conclusions. Mixtures of SDS and positively charged drugs form the same interesting phases as traditional catanionic mixtures. This may prove useful in obtaining functional controlled-release systems when using gels as drug carriers.
Similar content being viewed by others
References
H. Fukuda, K. Kawata, and H. Okuda. Bilayer-forming ion-pair amphiphiles from single-chain surfactants. J. Am. Chem. Soc. 112:1635-1637 (1990).
P. Jokela, B. Jönsson, and A. Kahn. Equilibria of catanionic surfactant–water systems. J. Phys. Chem. 91:3291-3298 (1987).
E. W. Kaler, A. K. Murthy, B. E. Rodriguez, and J. A. Zasadzinski. Spontaneous vesicle formation in aqueous mixtures of single-tailed surfactants. Science 245:1371-1374 (1989).
C. Tondre and C. Caillet. Properties of the amphiphilic films in mixed cationic/anionic vesicles: a comprehensive view from a literature analysis. Adv. Colloid Interface Sci. 93:115-134 (2001).
E. F. Marques, O. Regev, A. Khan, M. G. Miguel, and B. Lindman. Vesicle formation and general phase behaviour in the catanionic mixture SDS-DDAB-water. The anionic-rich side. J. Phys. Chem. B. 102:6746-6758 (1998).
E. F. Marques. Size and stability of catanionic vesicles: Effects of formation path, sonication, and aging. Langmuir 16:4798-4807 (2000).
L. L. Brasher and E. W. Kaler. A small-angle neutron scattering (SANS) contrast variation investigation of aggregate composition in catanionic surfactant mixtures. Langmuir 12:6270-6276 (1996).
J. M. Corkill, J. F. Goodman, S. P. Harrold, and J. R. Tate. Monolayers formed by mixtures of anionic and cationic surface-active agents. Trans. Faraday Soc. 63:247-256 (1996).
L. E. Bromberg. Enhanced nasal retention of hydrophobically modified polyelectrolytes. J. Pharm. Pharmacol. 53:109-114 (2001).
J. Carlfors, K. Edsman, R. Petersson, and K. Jörnving. Rheological evaluation of Gelrite in situ gels for ophthalmic use. Eur. J. Pharm. Sci. 6:113-119 (1998).
G. M. Grass and J. R. Robinson. Relationship of chemical structure to corneal penetration and influence of low viscosity solution on ocular bioavailability. J. Pharm. Sci. 73:1021-1027 (1984).
N. A. Peppas, P. Bures, W. Leobandung, and H. Ichikawa. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 50:27-46 (2000).
M. Paulsson and K. Edsman. Controlled drug release from gels using surfactant aggregates: II. Vesicles formed from mixtures of amphiphilic drugs and oppositely charged surfactants. Pharm. Res. 18:1586-1592 (2001).
R. M. Minardi, P. C. Schultz, and B. Vuano. Triangular phase diagram of the catanionic system dodecyltrimethylammonium bromide-disodium dodecanephosphonate-water. Colloids Surf. A. 197:167-172 (2002).
C. M. Wilkinson. Extended use of, and comments on, the drop-weight (drop-volume) technique for the determination of surface and interfacial tensions. J. Colloid Interface Sci. 40:14-26 (1972).
M. Almgren, K. Edwards, and G. Karlsson. Cryo transmission electron microscopy of liposomes and related structures. Colloid Surf. A-Physicochem. Eng. Asp. 174:3-21 (2000).
L. Bohlin. New instrumentation for advanced rheometry. Suppl. Rheol. Acta. 26:161-164 (1988).
W. I. Higuchi. Analysis of data on the medicament release from ointments. J. Pharm. Sci. 51:802-804 (1962).
P. L. Ritger and N. A. Peppas. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 5:23-26 (1987).
M. Paulsson and K. Edsman. Controlled drug release from gels using surfactant aggregates: I. Effect of lipophilic interactions for a series of uncharged substances. J. Pharm. Sci. 90:1216-1225 (2001).
A. Sein and J. B. F. N. Engberts. Micelle to lamellar aggregate transition of an anionic surfactant in dilute aqueous solution induced by alkali metal chloride and tetraalkylammonium chloride salts. Langmuir 11:455-465 (1995).
H. Hauser, N. Gains, and M. Muller. Vesiculation of unsonicated phospholipid dispersions containing phosphatidic acid by pH adjustment: Physicochemical properties of the resulting unilamellar vesicles. Biochemistry 22:4775-4781 (1983).
A. T. Florence, P. Arunothayanun, S. Kiri, M. S. Bernard, and I. F. Uchegbu. Some rheological properties of nonionic surfactant vesicles and the determination of surface hydration. J. Phys. Chem. B 103:1995-2000 (1999).
M. Paulsson and K. Edsman. Controlled drug release from gels using lipophilic interactions of charged substances with surfactants and polymers. J. Colloid Interface Sci. 248:194-200 (2002).
N. W. Taylor and E. B. Bagley. Dispersions or solutions? A mechanism for certain thickening agents. J. Appl. Polym. Sci. 18:2747-2761 (1974).
C. Caillet, M. Hebrant, and C. Tondre, Sodium octyl sulfate/cetyltrimethylammonium bromide catanionic vesicles: Aggregate composition and probe encapsulation. Langmuir 2000:9099-9102 (2000).
K. Östh, M. Paulsson, E. Björk, and K. Edsman. Evaluation of controlled drug release and absorption from gels on pig nasal mucosa in a horizontal Ussing chamber. J. Control. Release 83:377-388 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bramer, T., Paulsson, M., Edwards, K. et al. Catanionic Drug–Surfactant Mixtures: Phase Behavior and Sustained Release from Gels. Pharm Res 20, 1661–1667 (2003). https://doi.org/10.1023/A:1026103805283
Issue Date:
DOI: https://doi.org/10.1023/A:1026103805283