Abstract
Purpose. To determine the expression and functional activity of proton-coupled oligopeptide transporters (POT) in retinal pigment epithelial (RPE) cells.
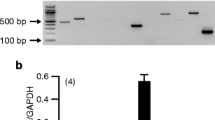
Methods. RT-PCR was used to probe the presence of POT mRNA in freshly isolated bovine RPE (BRPE) and human RPE (HRPE) cells, a human RPE cell line (ARPE-19), and human and bovine neural retina. [14C]GlySar uptake was used to characterize POT activity in cultured ARPE-19 cells and freshly isolated BRPE cell sheet suspensions.
Results. PHT1 mRNA was expressed in BRPE, HRPE, ARPE-19, and bovine and human neural retina. In contrast, PEPT2 and PHT2 were expressed only in bovine and human retina, and PEPT1 could not be detected. GlySar exhibited a linear uptake over 6 h at pH values of 6.0 and 7.4, with greater uptake at pH 7.4 (p < 0.01). GlySar uptake did not exhibit saturability (5-2000 μM) and was unchanged when studied in the presence of 1 mM L-histidine. In contrast, GlySar uptake was significantly decreased when studied at 4°C or in the presence of endocytic inhibitors at 37°C (p < 0.01). Studies in BRPE cell sheet suspensions validated the results obtained in ARPE-19 cells and strongly suggested the absence of POT on the apical and basolateral membranes of RPE.
Conclusions. PHT1 mRNA is present in native bovine and human RPE and a human RPE cell line. However, the data argue against PHT1 being expressed on plasma membranes of RPE. Overall, GlySar appears to be taken up by RPE cells via a low-affinity, endocytic process.
Similar content being viewed by others
References
B. A. Hughes, R. P. Gallenmore, and S. S. Miller. Transport mechanisms in the retinal pigment epithelium. In M. F. Marmor, T. J. Wolfenberger (eds.), The Retinal Pigment Epithelium Function and Disease, Oxford University Press, New York, 1998 pp. 103–135.
L. J. Rizzolo. Polarization of the Na+,K+–ATPase in epithelia derived from the neuroepithelium. Int. Rev. Cytol. 185:195–235 (1999).
R. Kannan, D. Tiang, J. Hu, and D. Bok. Glutathione transport in human retinal pigment epithelial (HRPE) cells: Apical localization of sodium–dependent GSH transport. Exp. Eye Res. 72:661–666 (2001).
C. D. Chancy, R. Kekuda, W. Huang, P. D. Prasad, J.–M. Kuhnel, F. M. Sirotnak, P. Roon, V. Ganapathy, and S. B. Smith. Expression and differential polarization of the reduced–folate transporter–1 and the folate receptor α in mammalian retinal pigment epithelium. J. Biol. Chem. 275:20676–20684 (2000).
J. V. Aukunuru, G. Sunkara, N. Bandi, W. B. Thoreson, and U. B. Kompella. Expression of multidrug resistance–associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a novel aldose reductase inhibitor. Pharm. Res. 18:565–572 (2001).
P. D. Rajan, R. Kekuda, C. D. Chancy, W. Huang, V. Ganapathy, and S. B. Smith. Expression of the extraneuronal monoamine transporter in RPE and neural retina. Curr. Eye Res. 20:195–204 (2000).
Y.–J. Fei, Y. Kanai, S. Nussberger, V. Ganapathy, F. H. Leibach, M. F. Romero, S. K. Singh, W. F. Boron, and M. A. Hediger. Expression cloning of a mammalian proton–coupled oligopeptide transporter. Nature 368:563–566 (1994).
F. H. Leibach and V. Ganapathy. Peptide transporters in the intestine and the kidney. Annu. Rev. Nutr. 16:99–119 (1996).
H. Daniel. Function and molecular structure of brush border membrane peptide/H+ symporters. J. Membr. Biol. 154:197–203 (1996).
H. Shen, D. E. Smith, T. Yang, Y. G. Huang, J. B. Schnermann, and F. C. Brosius III. Localization of PEPT1 and PEPT2 proton–coupled oligopeptide transporter mRNA and protein in rat kidney. Am. J. Physiol. 276:F658–F665 (1999).
W. Liu, R. Liang, S. Ramamoorthy, Y.–J. Fei, M. E. Ganapathy, M. A. Hediger, V. Ganapathy, and F. H. Leibach. Molecular cloning of PEPT 2, a new member of the H+/peptide cotransporter family, from human kidney. Biochim. Biophys. Acta 1235:461–466 (1995).
H. Daniel and M. Herget. Cellular and molecular mechanisms of renal peptide transport. Am. J. Physiol. 273:F1–F8 (1997).
U. V. Berger and M. A. Hediger. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat. Embryol. 199:439–449 (1999).
A. Novotny, J. Xiang, W. Stummer, N. S. Teuscher, D. E. Smith, and R. F. Keep. Mechanisms of 5–aminolevulinic acid uptake at the choroid plexus. J. Neurochem. 75:321–328 (2000).
C. Shu, H. Shen, N. S. Teuscher, P. J. Lorenzi, R. F. Keep, and D. E. Smith. Role of PEPT2 in peptide/mimetic trafficking at the blood–cerebrospinal fluid barrier: Studies in rat choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 301:820–829 (2002).
N. S. Teuscher, A. Novotny, R. F. Keep, and D. E. Smith. Functional evidence for the presence of PEPT2 in rat choroid plexus: Studies with glycylsarcosine. J. Pharmacol. Exp. Ther. 294:494–499 (2000).
N. S. Teuscher, R. F. Keep, and D. E. Smith. PEPT2–mediated uptake of neuropeptides in rat choroid plexus. Pharm. Res. 18:807–813 (2001).
T. Yamashita, S. Shimada, W. Guo, K. Sato, E. Kohmura, T. Hayakawa, T. Takagi, and M. Tohyama. Cloning and functional expression of a brain peptide/histidine transporter. J. Biol. Chem. 272:10205–10211 (1997).
K. Sakata, T. Yamashita, M. Maeda, Y. Moriyama, S. Shimada, and M. Tohyama. Cloning of a lymphatic peptide/histidine transporter. J. Biochem. 356:53–60 (2001).
L. C. Kreutz and M. R. Ackermann. Porcine reproductive and respiratory syndrome virus enters cells through a low pH–dependent endocytic pathway. Virus Res. 42:137–147 (1996).
M.–C. Lee, C. M. Cahill, J.–P. Vincent, and A. Beaudet. Internalization and trafficking of opioid receptor ligands in rat cortical neurons. Synapse 43:102–111 (2002).
M. Buraczynska, A. J. Mears, S. Zareparsi, R. Farjo, E. Filippova, Y. Yuan, S. P. MacNee, B. Hughes, and A. Swaroop. Gene expression profile of native human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 43:603–607 (2002).
M. M. Bradford. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72:248–254 (1976).
J. Hu and D. Bok. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis. 7:14–19 (2000).
C. Shu, H. Shen, U. Hopfer, and D. E. Smith. Mechanism of intestinal absorption and renal reabsorption of an orally active ACE inhibitor: Uptake and transport of fosinopril in cell cultures. Drug Metab. Dispos. 29:1307–1315 (2001).
M. Sugawara, W. Huang, Y.–J. Fei, F. H. Leibach, V. Ganapathy, and M. E. Ganapathy. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J. Pharm. Sci. 89:781–789 (2000).
C. W. Botka, T. W. Wittig, R. C. Graul, C. U. Nielsen, and W. Sadée. Human proton/oligopeptide transporter (POT) genes: Identification of putative human genes using bioinformatics. AAPS PharmSci 2(2): article 16(2000) (http://www.pharmsci.org).
H. Saito, H. Motohashi, M. Mukai, and K.–I. Inui. Cloning and characterization of a pH–sensing regulatory factor that modulates transport activity of the human H+/peptide cotransporter, PEPT1. Biochem. Biophys. Res. Commun. 237:577–582 (1997).
S. D. Freedman, H. F. Kern, and G. A. Scheele. Acinar lumen pH regulates endocytosis, but not exocytosis, at the apical plasma membrane of pancreatic acinar cells. Eur. J. Cell Biol. 75:153–162 (1998).
K.–D. Lee, S. Nir, and D. Papahadjopoulos. Quantitative analysis of liposome–cell interactions in vitro: Rate constants of binding and endocytosis with suspension and adherent J774 cells and human monocytes. Biochemistry 32:889–899 (1993).
K. C. Dunn, A. D. Marstein, V. L. Bonilha, E. Rodriguez–Boulan, F. Giordano, and L. M. Hjelmeland. Use of the ARPE–19 cell line as a model of RPE polarity: Basolateral secretion of FGF5. Invest. Ophthalmol. Vis. Sci. 39:2744–2749 (1998).
G. M. Holtkamp, M. van Rossem, A. F. de Vos, B. Willekens, R. Peek, and A. Kijlstra. Polarized secretion of IL–6 and IL–8 by human retinal pigment epithelial cells. Clin. Exp. Immunol. 112:34–43 (1998).
M. Thamotharan, Y. B. Lombardo, S. Z. Bawani, and S. A. Adibi. An active mechanism for completion of the final stage of protein degradation in the liver, lysosomal transport of dipeptides. J. Biol. Chem. 272:11786–11790 (1997).
X. Zhou, M. Thamotharan, A. Gangopadhyay, C. Serdikoff, and S. A. Adibi. Characterization of an oligopeptide transporter in renal lysosomes. Biochim. Biophys. Acta 1466:372–378 (2000).
M. Boulton and P. Dayhaw–Barker. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 15(Pt 3):384–389 (2001).
R. W. Young and D. Bok. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 42:392–402 (1969).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ocheltree, S.M., Keep, R.F., Shen, H. et al. Preliminary Investigation into the Expression of Proton-Coupled Oligopeptide Transporters in Neural Retina and Retinal Pigment Epithelium (RPE): Lack of Functional Activity in RPE Plasma Membranes. Pharm Res 20, 1364–1372 (2003). https://doi.org/10.1023/A:1025741723724
Issue Date:
DOI: https://doi.org/10.1023/A:1025741723724