Abstract
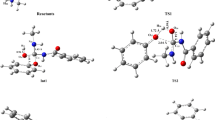
Dimethylcarbamoyl transfer from N-acyloxypyridinium salts to pyridine N-oxides in acetonitrile occurs in one stage by the forced concerted SN2 mechanism. The rate and equilibrium of the reaction are fairly described by the Brönsted equation. The Marcus equation provides a much higher quality of reactivity predictions.
Similar content being viewed by others
REFERENCES
Williams, A., Adv. Phys. Org. Chem. 1992, vol. 27, p. 1; Williams, A., Chem. Soc. Rev. 1994, vol. 23, no. 1, p. 93.
Jencks, W.P., Catalysis in Chemistry and Enzymology New York: McGraw-Hill, 1969. Translated under the title Kataliz v khimii i enzimologii Moscow: Mir, 1972, p. 467; Litvinenko L.M. and Oleinik N.M., Mekhanizmy deistviya organicheskikh katalizatorov (Mechanisms of Action of Organic Catalysts), Kiev: Naukova Dumka, 1984; Jencks, W.P., Chem. Rev., 1985, vol. 85, no. 6, p. 511.
Trushkov, I.V., Chuvylkin, N.D., Koz'min, A.S., and Zefirov, N.S., Izv. Ross. Akad. Nauk, Ser. Khim. 1995, no. 5, p. 804; Shaik, S.S., Schlegel, H.B., and Wolfe, S., Theoretical Aspects of Physical Organic Chemistry. The S N2 Mechanism, New York: Wiley, 1992.
Marcus, R.A., J. Phys. Chem. 1968, vol. 72, no. 12, p. 891; Bazilevskii, M.V., Koldobskii, S.G., and Tikhomirov, V.A., Usp. Khim. 1986, vol. 55, no. 10, p. 1667.
Denisov, E.T., Mendeleev Commun. 1992, vol. 2, no. 1, p. 1.
Lewis, E.S., J. Phys. Chem. 1986, vol. 90, no. 16, p. 3756.
Kresge, A.J., Chem. Soc. Rev. 1973, vol. 2, no. 4, p. 457.
Kreevoy, M.M. and Lee, I.-S.H., J. Am. Chem. Soc. 1984, vol. 106, no. 9, p. 2550.
Rybachenko, V.I., Shreder, G., Chotii, K.Yu., Semenova, R.G., Grebenyuk, L.V., Lenska, B., Kovalenko, V.V., and Rozvadovskii, Z., Zh. Obshch. Khim. 1998, vol. 68, no. 1, p. 120; Rybachenko, V.I., Shreder, G., Chotii, K.Yu., and Kovalenko, V.V., Teor. Eksp. Khim. 1998, vol. 34, no. 2, p. 96; Rybachenko, V.I., Shreder, G., Chotii, K.Yu., Kovalenko, V.V., Lenska, B., and Red'ko, A.N., Teor. Eksp. Khim. 2000, vol. 36, no. 6, p. 363.
Savelova, V.A. and Oleinik, N.M., Mekhanizmy deistviya organicheskikh katalizatorov (Mechanisms of Action of Organic Catalysts), Kiev: Naukova Dumka, 1990; Pal'm, V.A., Osnovy kolichestvennoi teorii organicheskikh reaktsii (Fundamentals of Quantitative Theory of Organic Reactions), Leningrad: Khimiya, 1977.
Schmid, R. and Sapunov, V.N., Non-Formal Kinetics Weinheim: Chemie, 1982.
Lenska, B., Hes, M., and Schroeder, G., Acta Chim. Hung. 1994, vol. 131, no. 5, p. 671.
Titov, E.V., Makarova, R.A., Rybachenko, V.I., Chotii, K.Yu., and Goncharova, L.D., Teor. Eksp. Khim. 1988, vol. 24, no. 2, p. 227.
Rybachenko, V.I., Shreder, G., Titov, E.V., Chotii, K.Yu., Semenova, R.G., and Makarova, R.A., Zh. Obshch. Khim. 1996, vol. 66, no. 6, p. 1007.
Rybachenko, V.I., Shreder, G., Lenska, B., Chotii, K.Yu., Semenova, R.G., Makarova, R.A., and Grebenyuk, L.V., Teor. Eksp. Khim. 1997, vol. 33, no. 3, p. 143.
Semenova, R.G., Gol'dshtein, I.P., Grebenyuk, L.V., and Rybachenko, V.I., Zh. Fiz. Khim. 1997, vol. 71, no. 2, p. 248.
Jedrzejczak, M., Motie, R.E., Satchel, D.P.N., Satchel, S.S., and Wassef, W.N., J. Chem. Soc., Perkin Trans. 2 1994, no. 7, p. 1471.
More O'Ferrall, R.A., J. Chem. Soc. B 1970, no. 2, p. 274.
Jencks, D.A. and Jencks, W.P., J. Am. Chem. Soc. 1977, vol. 94, no. 24, p. 7498.
Ba-Saif, S.A., Waring, M.A., and Williams, A., J. Chem. Soc., Perkin Trans. 2 1991, no. 10, p. 1653.
Ba-Saif, S.A., Colthurst, M., Waring, M.A., and Williams, A., J. Chem. Soc., Perkin Trans. 2 1991, no. 11, p. 1901.
Lewis, E.S., Bull. Soc. Chim. Fr. 1988, no. 2, p. 259.
Albery, W.J. and Kreevoy, M.M., Adv. Phys. Org. Chem. 1978, vol. 16, p. 87.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schroeder, G., Rybachenko, V.I., Chotii, K.Y. et al. Rate and Equilibrium Constants of Dimethylcarbamoyl Transfer between Pyridine N-Oxides. Russian Journal of General Chemistry 73, 455–462 (2003). https://doi.org/10.1023/A:1024974323588
Issue Date:
DOI: https://doi.org/10.1023/A:1024974323588