Abstract
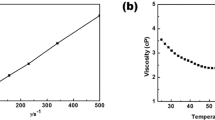
γ-Butyrolactone-ethylene carbonate (BL-EC) mixtures have been investigated as electrolytes for Li-ion batteries using LiPF6 and LiBF4 as lithium salt. The thermal stability of the electrolytes in a large range of temperatures (−90 °C to 40 °C) have been studied by differential scanning calorimetry (DSC) and X-ray diffraction (XRD). From the results of these experiments, the phase diagram of the BL-EC system has been determined. It is characterised by a eutectic point at −56.3 °C and a molar fraction in EC: x EC = 0.1. A metastable compound has been demonstrated below −90 °C at x EC = 0.4. Conductivity measurements of BL-EC solutions, in the presence of LiPF6 and LiBF4, indicate that LiPF6 in the eutectic mixture is the most conducting electrolyte in the range of temperatures investigated (−30 °C to room temperature). Nevertheless, at low temperature, LiBF4 based electrolytes compete well with LiPF6, especially when the amount of EC in the mixture is as high as x EC = 0.5. Moreover, recrystallisation of the salt below −20 °C is avoided when LiBF4 is used as salt. A large increase in viscosity of the solvent mixture is observed when a salt is added, but the increase is lower for LiBF4 than LiPF6. When EC is added to BL at constant salt concentration (1 M), the conductivity of LiPF6 solutions decreases more rapidly than LiBF4 solutions. This has been attributed, at least partially, to the dissociating power of EC. The electrochemical windows of BL-EC (equimolar) mixtures in the presence of LiPF6 and LiBF4 are comparable but it is shown that the solvents oxidation rate at high potentials is lower when LiBF4 is used.
Similar content being viewed by others
References
G.-C. Chung, Electrochem. Commun. 1 (1999) 493.
S.-I. Tobishima and J.-I. Yamaki, J. Power Sources 81 (1999) 882.
A. Chagnes, B. Carré, P. Willmann and D. Lemordant, Electrochim. Acta 46 (2001) 1783.
A. Chagnes, B. Carré, P. Willmann and D. Lemordant, J. Power Sources 109 (2002) 203.
R. Naejus, D. Lemordant and R. Coudert, J. Chem. Thermodyn. 29 (1997) 1503.
A. Moumouzia and G. Panapoulos, J. Chem. Eng. Data 36 (1991) 20.
M. Winter and J.O. Besenhard, in M. Wakihara and O. Yamamoton (Eds), 'Lithium Ion Batteries: Fundamentals and Performance', (Wiley-VCH, New York, 1999).
J.O. Besenhard and H.P. Fritz, J. Electroanal. Chem. 53 (1974) 329.
J.O. Besenhard, M. Winter, J. Yang and W. Biberacher, J. Power Sources 54 (1995) 228.
M.C. Smart, B.V. Ratnakumar and S. Surampudi, J. Electrochem. Soc. 146 (1999) 486.
M.S. Ding, K. Xu and T.R. Jow, J. Electrochem. Soc 147 (2000) 1688.
M.S. Ding, K. Xu and T.R. Jow, J. Electrochem. Soc. 148 (2001) A299.
A. Chagnes, B. Carré, V. Agafonov, P. Willmann and D. Lemordant, J. Phys. IV, 11 (Pr 10, XXVII JEEP, Journe´es d'Etude des Equilibres entre Phases 2001) 27.
D. Giron, Electrochim. Acta 248 (1995) 1.
J.F. Kincaid, H. Eyring and A.E. Stearn, Chem. Rev. 28 (1941) 301.
K. Hayamizu, Solid State Ionics 107 (1998) 1.
I. Geoffroy, P. Willmann, K. Mesfar, B. Carre´ and D. Lemordant, Electrochim. Acta 45 (2000) 2019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chagnes, A., Allouchi, H., Carré, B. et al. γ-Butyrolactone-ethylene carbonate based electrolytes for lithium-ion batteries. Journal of Applied Electrochemistry 33, 589–595 (2003). https://doi.org/10.1023/A:1024904918401
Issue Date:
DOI: https://doi.org/10.1023/A:1024904918401