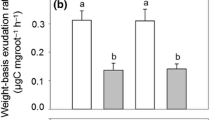
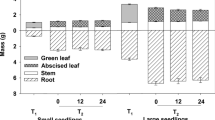
Abstract
We studied the source of the nitrogen used for the growth and resprouting of holm-oak (Quercus ilexL.), and the contribution of nitrogen and carbohydrate root reserves to these processes. Three-year-old plants were grown in a greenhouse with either a sufficient or restricted nitrogen supply for one year. Half the individuals were subjected to shoot excision to provoke resprouting, and a 15N solution was given to these plants and to controls for two months. Nitrogen, Total Non-structural Carbohydrate (TNC), Total Soluble Protein content, and 15N and 13C composition were determined, and histological analyses of woody tissue were performed. Our results show that N-deprived plants used nitrogen from root reserves to support a growth rate similar to that of non-deprived plants. However, deprived plants lost their resprouting capacity in spite of the high TNC accumulation and nitrogen resupply to the soil. After the supply of nitrogen was restored to N-deprived plants, this nutrient mainly accumulated in under-ground organs, which limited the above-ground growth. Resprouting plants first remobilized the nitrogen stored in roots, and thereafter took it up from the solution. The root-crown region did not behave as a specialised reserve organ in three-year-old Quercus ilex L. plants.
Similar content being viewed by others
References
Adams M B, Allen H L and Davey C B 1986 Accumulation of starch in roots and foliage of loblolly pine (Pinus taeda L.): effects of season, site and fertilization. Tree Physiol. 2, 35–46.
Améziane R, Richard-Molard C, Deleens E, Morot-Gaudry J F and Limami A M 1997 Nitrate (15NO3) limitation affects nitrogen partitioning between metabolic and storage sinks and nitrogen reserve accumulation in chicory (Cichorium intybus L.). Planta 202, 303–312.
Azcón-Bieto J and Osmond C B 1983 Relationship between photosynthesis and respiration. The effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol. 71, 574–581.
Bradford M M 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann. Biochem. 72, 248–254.
Canadell J, Lloret F and López-Soria L 1991. Resprouting vigour of two mediterranean shurb species after experimental fire treatments. Vegetatio 95, 119–126.
Canadell J and López-Soria L 1998 Root crown reserves support regrowth following clipping of two Mediterranean shrubs. Funct. Ecol. 12, 31–38.
Canadell J, Djema A, López B, Lloret F, Sabaté S, Siscart D and Gracia C A 1999 Structure dynamics of the root system. In Ecology of Mediterranean Evergreen Oak Forests. Eds. F Rodà, J Retana, C A Gracia and J Bellot, pp. 47–59. Springer-Verlag, Berlin.
Carr D J, Carr S G M and Janker R 1982 The eucalypt lignotuber: a position-dependent organ. Ann. Bot. 50, 481–489.
Fircks Y V and Forsse L S 1998 Seasonal fluctuations of starch in root and stem tissues of coppiced Salix viminalis plants grown under two nitrogen regimes. Tree Physiol. 18, 243–249.
Fleck I, Grau D, Sanjosé M and Vidal D 1996a Carbon isotope discrimination in Quercus ilex resprouts after fire and tree-fell. Oecologia 105, 286–292.
Fleck I, Grau D, Sanjosé M and Vidal D 1996b Influence of fire and tree-fell on physiological parameters in Quercus ilex resprouts. Ann. Sci. For. 53, 337–346.
Fleck I, Hogan K P, Llorens L, Abadía A and Aranda X 1998 Photosynthesis and photoprotection in Quercus ilex resprouts after fire. Tree Physiol. 18, 607–614.
Granato T C, Raper C D Jr and Wilkerson G G 1989 Respiration rate in maize roots is related to concentration of reduced nitrogen and proliferation of lateral roots. Physiol. Plant. 76, 419–424.
Kauppi A and Rinne P 1987 Initiation, structure and sprouting of dormant basal buds in Betula pubescens. Flora 179, 55–83.
Kruger E L and Reich P B 1997 Response of hardwood regeneration to fire in mesic forest openings. II Leaf gas exchange, nitrogen concentration, and water status. Can. J. For. Res. 27, 1832–1840.
Malanson G P and Trabaud L 1988 Vigour of post-fire resprouting by Quercus coccifera L. J. Ecol. 76, 351–365.
Millard P and Neilsen G H 1989 The influence or nitrogen supply on the uptake and remobilization of stored N for the seasonal growth of apple trees. Ann. Bot. 63, 301–309.
Millard P and Proe M F 1991 Leaf demography and the seasonal internal cycling of nitrogen in sycamore (Acer pseudoplatanus L.) seedlings in relation to nitrogen supply. New Phytol. 117, 587–596.
Millard P 1996. Ecophysiology of the internal cycling of nitrogen for tree growth. J. Plant Nutr. Soil Sc. 159, 1–10.
Molinas M L and Verdaguer D 1993a Lignotuber ontogeny in the cork-oak (Quercus suber; Fagaceae) I. Late embryo. Am. J. Bot. 80, 172–181.
Molinas M L and Verdaguer D 1993b Lignotuber ontogeny in the cork-oak (Quercus suber; Fagaceae) II. Germination and young seedling. Am. J. Bot. 80, 182–191
Neilsen D, Millard P, Neilsen G H and Hogue E J 1997 Sources of N for leaf growth in a high density apple (Malus domestica). Tree Physiol. 17, 733–739.
Oechel W C and Hastings S J 1983 The effects of fire on photosynthesis in chaparral resprouts. In Ecological Studies Vol. 43, Mediterranean Type Ecosystems. Eds. F J Kruger, D T Mitchell and U M Jarvis. pp. 274–285. Springer Verlag. Berlin.
Pascual G, Molinas M and Verdaguer D 2002 Comparative anatomical analysis of the cotyledonary region in three Mediterranean Basin Quercus (Fagaceae). Am. J. Bot. 89, 383–392
Poorter H 1989 Interspecific variation in relative growth rate: on ecological causes and physiological consequences. In Causes and Consequences of Variations in Growth Rate and Productivity of Higher Plants. Eds. H Lambers, M L Cambridge, H Konings and T L Pons. pp. 45–68. SPB Academic Publishing. The Hague.
Retana J, Riba J, Castell C and Espelta J M 1992 Regeneration by sprouting of holm-oak (Quercus ilex) stands exploited by selection thinning. Vegetatio 99–100, 355–364.
Saruwatari M W and Davis S D 1989 Tissue water relations of three chaparral shrubs species after wild-fire. Oecologia 80, 303–308.
Tagliavini M, Millard P and Quartieri M 1998 Storage of foliar-absorbed nitrogen and remobilization for spring growth in young nectarine (Prunus persica var. nectarina) trees. Tree Physiol. 18, 201–207.
Talouizte A, Champigny M L, Bismuth E and Moyse A 1984 Root carbohydrate metabolism associated with nitrate assimilation in wheat previously deprived of nitrogen. Physiol. Vég. 22, 19–27.
Tiedeman A R, Clary W P and Barbour R J 1987 Under-ground systems of gamble-oak Quercus gambelii in central Utah. Am. J. Bot. 74, 1065–1071.
Tolley Henry L and Raper D Jr 1991 Soluble carbohydrates allocation to roots, photosynthetic rate of leaves, and nitrate assimilation as affected by nitrogen stress and irradiance. Bot. Gaz. 152: 23–33.
Tredici P D 1992 Natural regeneration of Ginkgo biloba from downward growing cotyledonary buds (basal chichi). Am. J. Bot. 79, 522–530.
Tromp J 1983 Nutrient reserves in roots of fruits trees, in particular carbohydrates and nitrogen. Plant Soil 71, 401–413.
Van der Werf A and Nagel OW 1996 Carbon allocation to shoots and roots in relation to nitrogen supply is mediated by cytokinins and sucrose: Opinion. Plant Soil 185, 21–32.
Verdaguer D and Molinas M 1997 Development and ultrastructure of the endodermis in the primary root of cork-oak (Quercus suber). Can. J. Bot. 75, 769–780.
Verdaguer D, García-Berthou E, Pascual G and Puigderrajols P 2001 Sprouting of seedlings of three Quercus species (Q. humilis, Q. ilex, and Q. suber L.) in relation to repeated pruning and the cotyledonary node. Aust. J. Bot. 49, 67–74.
Wendler R, Carvalho P O, Pereira J S and Millard P 1995 Role of nitrogen remobilization from old leaves for new leaf growth of Eucalyptus globulus seedlings. Tree Physiol. 15, 679–683.
Wiesler F, Dickmann J and Horst W J 1997 Effects of nitrogen supply on growth and nitrogen uptake by Miscanthus sinensis during establishment. Z. Pflanzenernähr. Bodenkd. 160, 25–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Omari, B., Aranda, X., Verdaguer, D. et al. Resource remobilization in Quercus ilex L. resprouts. Plant and Soil 252, 349–357 (2003). https://doi.org/10.1023/A:1024792206369
Issue Date:
DOI: https://doi.org/10.1023/A:1024792206369