Abstract
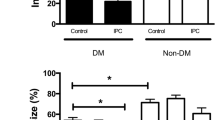
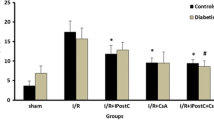
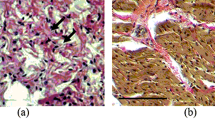
The sympathetic nervous systems may bear relevance to the increased incidence of heart failure in diabetes (DM). In our isolated rat hearts perfused at constant low flow rate, norepinephrine dose-dependently enhanced diabetic myocardial damage, particularly during underperfusion. The purpose of this investigation is to examine the effects of epinephrine on the ischemic injury and on the reperfusion injury in DM and non-DM rat hearts, and to clarify whether the cardiac states during underperfusion at constant low pressure are similar to those at constant low flow rate. Isolated streptozotocin-induced 6-week DM and non-DM rat hearts with a balloon in the left ventricle (LV) were paced and normal perfused at 75 cm H2O with normoxic Krebs-Henseleit solution. Then the hearts were underperfused at 35 cm H2O, a constant low pressure with below one-third of the pre-ischemic coronary perfusion flow (CPF) level. Four min after the start of underperfusion, the perfusate was changed to that containing epinephrine 10−6 M. After 45 min underperfusion with or without epinephrine, all of the hearts were reperfused without epinephrine at 75 cm H2O for 45 min. To detect changes in LV stiffness, the isometric tension along the longitudinal direction of the whole heart and the LV isovolumic pressure were monitored simultaneously. In DM hearts, the underperfusion alone caused a slight increase in LV stiffness, and all the changes recovered to the pre-ischemic levels during reperfusion. Epinephrine during underperfusion accelerated the start of increase in LV stiffness and the decrease in CPF. During reperfusion the changes recovered partly to the control levels. In non-DM hearts, epinephrine during underperfusion caused only a slight increase in LV stiffness though a similar low CPF to DM hearts. However, the reperfusion caused a marked increase in LV stiffness and a lower recovery of CPF. Epinephrine at constant low pressure, as well as norepinephrine at constant low flow rate, enhanced the ischemic injury, particularly in DM hearts, while aggravated the reperfusion injury in non-DM hearts.
Similar content being viewed by others
References
Schömig A, Dart AM, Dietz R, Mayer E, Kübler W: Release of endogenous catecholamines in the ischemic myocardium of the rat: Part A: Locally mediated release. Circ Res 55: 689-701, 1984
Higuchi M, Asakawa T: Effects of nitroglycerin on regional myocardial function in the underperfused canine heart. J Cardiovasc Pharmacol 7: 1087-1095, 1985
Jaffe AS, Spadaro JJ, Schechtman K, Roberts R, Geltman EM, Sobel BE: Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J 108: 31-37, 1984
Vered Z, Battler A, Segal P, Liberman D, Yerushalmi Y, Berezin M, Neufeld HN: Exercise-induced left ventricular dysfunction in young men with asymptomatic diabetes mellitus (diabetic cardiomyopathy). Am J Cardiol 54: 633-637, 1984
Gøtzsche O: Abnormal myocardial calcium uptake in streptozocin-diabetic rats. Evidence for a direct insulin effect on catecholamine sensitivity. Diabetes 34: 287-290, 1985
Higuchi M, Ikema S, Matsuzaki T, Hirayama K, Sakanashi M: Effects of norepinephrine on hypoperfusion-reperfusion injuries in hearts isolated from normal and diabetic rats. J Mol Cell Cardiol 23: 137-148, 1991
Palik I, Koltai MZ, Kolonics I, Wagner M, Pogatsa G: Effects of coronary occlusion and norepinephrine on the myocardium of alloxan-diabetic dogs. Basic Res Cardiol 77: 499-506, 1982
Friedman JJ: Vascular sensitivity and reactivity to norepinephrine in diabetes mellitus. Am J Physiol 256: H1134-H1138, 1989
Pierce GN, Dhalla NS: Sarcolemmal Na+,K+-ATPase activity in diabetic rat heart. Am J Physiol 245: C241-C247, 1983
Heyliger CE, Prakash A, McNeill JH: Alterations in cardiac sarcolemmal Ca2+ pump activity during diabetes mellitus. Am J Physiol 252: H540-H544, 1987
Makino N, Dhalla KS, Elimban V, Dhalla NS: Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol 253: H202-H207, 1987
Pierce GN, Ramjiawan B, Dhalla NS, Ferrari R: Na+-H+ exchange in cardiac sarcolemmal vesicles isolated from diabetic rats Am J Physiol 258: H255-H261, 1990
Kusama Y, Hearse D, Avkiran M: Diabetes and susceptibility to reperfusion-induced ventricular arrhythmias. J Mol Cell Cardiol 24: 411-421, 1992
Higuchi M, Ikema S, Sakanashi M: Correlation of contractile dysfunction and abnormal tissue energy metabolism during hypoperfusion with norepinephrine in isolated rat hearts: Differences between normal and diabetic hearts. J Mol Cell Cardiol 24: 1125-1141, 1992
Higuchi M, Miyagi K, Nakasone J, Sakanashi M: Role of high glycogen in underperfused diabetic rat hearts with added norepinephrine. J Cardiovasc Pharmacol 26: 899-907, 1995
Hoh JFY, Rossmanith GH, Kwan LJ, Hamilton AM: Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random. Circ Res 62: 452-461, 1988
Saeki Y, Shiozawa K, Yanagisawa K, Shibata T: Adrenaline increases the rate of cross-bridge cycling in rat cardiac muscle. J Mol Cell Cardiol 22: 453-460, 1990
Hearse DJ, Stewart DA, Chain EB: Diabetes and the survival and recovery of the anoxic myocardium. J Mol Cell Cardiol 7: 397-415, 1975
Feuvray D, Idell-Wenger JA, Neely JR: Effects of ischemia on rat myocardial function and metabolism in diabetes. Circ Res 44: 322-329, 1979
Bricknell OL, Daries PS, Opie LH: A relationship between adenosine triphosphate, glycolysis and ischaemic contracture in the isolated rat heart. J Mol Cell Cardiol 13: 941-945, 1981
Penpargkul S, Schaible T, Yipintsoi T, Scheuer J: The effect of diabetes on performance and metabolism of rat hearts. Circ Res 47: 911-921, 1980
Chattou S, Coulombe A, Diacono J, Le Grand B, John G, Feuvray D: Slowly inactivating component of current in ventricular myocytes is decreased by diabetes and partially inhibited by known Na(+)-H(+)exchange blockers. J Mol Cell Cardiol 32: 1181-1192, 2000
Imahashi K, Hashimoto K, Yamaguchi H, Nishimura T, Kusuoka H: Alteration of intracellular Na+ during ischemia in diabetic rat hearts: The role of reduced activity in Na+/H+ exchange against stunning. J Mol Cell Cardiol 30: 509-517, 1998
Tani M, Neely JR: Hearts from diabetic rats are more resistant to in vitro ischemia: Possible role of altered Ca2+ metabolism. Circ Res 62: 931-940, 1988
Bielefeld DR, Pace CS, Boshell BR: Altered sensitivity of chronic diabetic rat heart to calcium. Am J Physiol 245: E560-E567, 1983
Haider B, Ahmed SS, Moschos CB, Oldewurtel HA, Regan TJ: Myocardial function and coronary blood flow response to acute ischemia in chronic canine diabetes. Circ Res 40: 577-583, 1977
Cross CR, Rieben PA, Salisbury PF: Influence of coronary perfusion and myocardial edema on pressure-volume diagram of left ventricle. Am J Physiol 201: 102-108, 1961
Vogel WM, Apstein CS, Briggs LL, Gaasch WH, Ahn J: Acute alterations in left ventricular diastolic chamber stiffness: Role of the ‘erectile’ effect of coronary arterial pressure and flow in normal and damaged hearts. Circ Res 51: 465-478, 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Higuchi, M., Hirata, K., Yamashita, A. et al. Effects of epinephrine on underperfusion-reperfusion injuries in diabetic and non-diabetic rat hearts. Mol Cell Biochem 248, 157–163 (2003). https://doi.org/10.1023/A:1024144520596
Issue Date:
DOI: https://doi.org/10.1023/A:1024144520596