Abstract
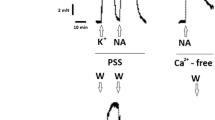
Using a strain measurement technique, we studied the mechanisms of the effect of a nitric oxide (NO) donor, nitroglycerin (NG), on contractions of smooth muscles of the main pulmonary artery of the rabbit induced by phenylephrine and caffeine in normal Krebs solution (NKS) or in nominally calcium-free solution (NCFS). Phenylephrine applications caused contractions consisting of an initial fast phasic low-amplitude component followed by a tonic higher-amplitude component. After caffeine-induced monophasic low-amplitude contraction, tension of the smooth muscle strip shifted below the conventional zero. Addition of NG to NKS resulted in a decrease in the smooth muscle tension below the conventional zero. Under the influence of NG, the initial phasic component of phenylephrine-induced contraction was partially suppressed, whereas the next tonic component was suppressed to a greater extent. At the same time, NG exerted nearly no influence on the amplitude of caffeine-induced contractions. Washing out by NKS of phenylephrine dissolved in NCFS resulted in initiation of a fast phasic high-amplitude contraction. Such a contraction did not develop either in the presence of NG or phenylephrine in NCFS or in the case of washing out of caffeine dissolved in NCFS. Our findings allow us to conclude that phenylephrine or caffeine added to the superfusate induce contractions of the smooth muscle cells (SMC) of the main pulmonary artery of the rabbit due to activation of Ca2+ release from the respective intracellular calcium stores. In addition, calcium ions entering SMC through the calcium channels of the plasma membrane are also involved in activation of the phenylephrine-induced contraction. The inhibitory effect of NG on the phenylephrine-induced contraction is related to the influence of NO on the release of Ca2+ from the inositol trisphosphate-sensitive intracellular calcium store and receptor-operated inflow of Ca2+ to SMC. Nitroglycerin did not significantly influence the caffeine-induced contraction and, therefore, Ca2+ release from the caffeine-sensitive store.
Similar content being viewed by others
REFERENCES
M. F. Shuba, N. I. Gokina, and A. V. Gurkovskaya, Mechanisms Underlying Excitation and Contractions of the Brain Vascular Smooth Muscles [in Russian], Naukova Dumka, Kyiv (1991).
M. F. Shuba and N. G. Kochemasova, Physiology of the Vascular Smooth Muscles [in Russian], Naukova Dumka, Kyiv (1988).
A. R. Butler, F. W. Flitney, and L. H. Williams, “NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist's perspecive, ” TiPS, 16, 18–22 (1995).
A. V. Zima, A. E. Belevich, A. V. Povstyan, et al., “Mechanism of action of nitric oxide donors on voltage-activated calcium channels in vascular smooth muscle cells, ” Neirofiziologiya/Neurophysiology, 28, No. 6, 296–304 (1996).
M. F. Shuba, A. E. Belevich, A. V. Gurkovskaya, and A. V. Zima, “Membrane and intracellular mechanisms of nitric oxide relaxing action on vascular smooth muscle, ” J. Vascul. Res., 33, 46 (1996).
L. A. Blatter and W. G. Wier, “Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current, ” Cell Calcium, 15, 122–131 (1994).
L. S. Archer, J. M. C. Huang, K. E. Weir, et al., “Nitric oxide and cGMP cause vasorelaxation by activation of a charibdotoxin-sensitive K channel by cGMP-dependent protein kinase, ” Proc. Natl. Acad. Sci. USA, 91, 7583–7587 (1994).
V. M. Bolotina, S. Najibi, J. J. Palacino, et al., “Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle, ” Nature, 368, 850–853 (1994).
R. A. Cohen, R. M. Weisbrod, M. Gericke, et al., “Mechanism of nitric oxide-induced vasodilatation. Refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx, ” Circ. Res., 84, 210–219 (1999).
J. Ahler, L. K. Axelsson, P. Karczewski, and R. G. G. Andersson, “Studies of the effect of glyceryl trinitrate and cyclic GMP on calcium turnover in bovine mesenteric artery, ” Pharmacol. Toxicol., 66, 277–282 (1990).
J. Ji, C. G. Benishin, and P. K. T. Pang, “Nitric oxide selectively inhibits intracellular Ca++ release elicited by inositol triphosphate but not caffeine in rat vascular aortic smooth muscle, ” J. Pharmacol. Exp. Ther., 285, No. 1, 16–21 (1998).
X.-L. Chen and C. M. Rembold, “Nitroglycerin relaxes rat tail artery primarily by lowering Ca2+ sensitivity and partially by repolarization, ” Am. J. Physiol., 271, H962-H968 (1996).
E. Robinson and A. Hudson, “Adrenoceptor pharmacology, ” Tocris Rev., Nov., No. 8, 1–6 (1998) (www.tocris.com/ TSframeset.html).
Y. M. Bae, M. K. Park, S. H. Lee, et al., “Contribution of Ca2+-activated K+ channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit, ” J. Physiol., 514, No. 3, 747–758 (1999).
J. N. Wells and J. G. Hardman, “Cyclic nucleotide phosphodiesterases, ” Adv. Cyclic Nucleotide Res., 8, 119–143 (1977).
C. Watanabe, H. Yamamoto, K. Hirano, et al., “Mechanisms of caffeine-induced contraction and relaxation of rat aortic smooth muscle, ” J. Physiol., 456, 193–213 (1992).
M. A. Noguera and M. P. D'Ocon, “Different and common intracellular calcium stores mobilized by noradrenaline and caffeine in vascular smooth muscle, ” N. Sch. Arch. Pharmacol., 345, No. 3, 333–341 (1992).
L. A. Blatter and W. G. Wier, “Agonist-induced [Ca2+]i waves and Ca2+-induced Ca2+-release in mammalian vascular smooth muscle cells, ” Am. J. Physiol., 263, No. 2, H576-H586 (1992).
H. Hamada, D. S. Damron, S. J. Hong, et al., “Phenylephrine-induced Ca2+ oscillations in canine pulmonary artery smooth muscle cells, ” Circ. Res., 81, 812–823 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Telezhkin, V.S., Shuba, M.F. Mechanisms Underlying Nitroglycerin-Induced Suppression of Phenylephrine- and Caffeine-Evoked Contractions of Smooth Muscles of the Rabbit Main Pulmonary Artery. Neurophysiology 35, 1–6 (2003). https://doi.org/10.1023/A:1023996804100
Issue Date:
DOI: https://doi.org/10.1023/A:1023996804100