Abstract
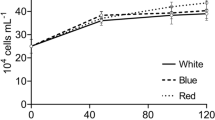
The unicellular green alga Haematococcus pluvialis accumulates large amounts of the red ketocarotenoid astaxanthin when exposed to various stress situations such as salt stress and high light intensities. Here, the light regulation of Haematococcus carotenoid biosynthesis was examined. Isolation and characterization of the lycopene β cyclase gene involved in carotenoid biosynthesis was carried out using a functional complementation approach. Subsequently, gene expression of lycopene cyclase, phytoene synthase, phytoene desaturase and carotenoid hydroxylase was analysed in green flagellate cells. All four genes revealed higher transcript levels in response to increased illumination. Not only the induction of astaxanthin biosynthesis but also carotenoid gene expression was found to be correlated with the redox state of the photosynthetic electron transport. In accordance with this result, increased transcript levels for carotenoid biosynthesis genes were detected under both blue and red light conditions. The application of different inhibitors of the photosynthetic electron flow indicated that the photosynthetic plastoquinone pool functions as the redox sensor for the up-regulation of carotenoid biosynthesis genes. These results suggested that in Haematococcus not only the specific astaxanthin pathway but also general carotenoid biosynthesis is subject to photosynthetic redox control.
Similar content being viewed by others
References
Allen, J.F., Alexciev, K. and Hakansson, G. 1995. Photosynthesis. Regulation by redox signalling. Curr. Biol. 5: 869–872.
Allen, J.F. and Pfannschmidt, T. 2000. Balancing the two photosystems: photosynthetic electron transfer governs transcription of reaction centre genes in chloroplasts. Phil. Trans. R. Soc. Lond. B 355: 1351–1357.
Böger, P., Sandmann, G. and Miller, R. 1981. Herbicide resitance in a mutant of the microalga Bummilleriopsisi filiformis. Photosynth. Res. 2: 61–74.
Bohne, F. and Linden, H. 2002. Regulation of carotenoid biosynthesis in response to light in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1579: 26–34.
Boussiba, S. 2000. Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol. Plant. 108: 111–117.
Boussiba, S., Fan, L. and Vonshak, A. 1992. Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. Meth. Enzymol. 213: 386–391.
Breitenbach, J., Misawa, N., Kajiwara, S. and Sandmann, G. 1996. Expression in Escherichia coli and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol. Lett. 140: 241–246.
Cunningham, F.X. and Gantt, E. 1998. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 557–583.
Cunningham, F.X., Pogson, B., Sun, Z., McDonald, K.A., DellaPenna, D. and Gantt, E. 1996. Functional analysis of the β and ε lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 8: 1613–1626.
Davies, B.H. 1976. Carotenoids. In: T.W. Goodwin (Ed.) Chemistry and Biochemistry of Plant Pigments, Academic Press, London, pp. 38–165.
Demmig-Adams, B., Gilmore, A.M. and Adams, W.W. 1996. In vivo functions of carotenoids in higher plants. FASEB J. 10: 403–412.
Dixon, M. and Webb, E.C. 1979. Enzymes. Academic Press, New York.
El Bissati, K. and Kirilovsky, D. 2001. Regulation of psbA and psaE expression by light quality in Synechocystis species PCC 6803. A redox control mechanism. Plant Physiol. 125: 1988–2000.
Emanuelssen, Nielsen, H. and von Heijne, G. 1999. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 8: 978–984.
Escoubas, J.M., Lomas, M., LaRoche, J. and Falkowski, P.G. 1995. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc. Natl. Acad. Sci. USA 92: 10237–10241.
Fabregas, J., Dominguez, A., Regueiro, M., Maseda, A. and Otero, A. 2000. Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 53: 530–535.
Fan, L., Vonshak, A., Gabbay, R., Hirschberg, J., Cohen, Z. and Boussiba, S. 1995. The biosynthetic pathway of astaxanthin in a green alga Haematococcus pluvialis as indicated by inhibition with dephenylamine. Plant Cell Physiol. 36: 1519–1524.
Fan, L., Vonshak, A., Zarka, A. and Boussiba, S. 1998. Does astaxanthin protect Haematococcus against light damage? Z. Naturforsch. 53: 93–100.
Fraser, P.D., Shimada, H. and Misawa, N. 1998. Enzymic confirmation of reactions involved in routes to astaxanthin formation, elucidated using a direct substrate in vitro assay. Eur. J. Biochem. 252: 229–236.
Goodwin, T.W. 1980. The Biochemistry of Carotenoids. Chapman-Hall, New York.
Holden, H. 1976. Chlorophylls. In: T.W. Goodwin (Ed.) Chemistry and Biochemistry of Plant Pigments, Academic Press, New York, vol. 2, pp. 1–37.
Humbeck, K., Hoffmann, B. and Senger, H. 1988. Influence of energy flux and quality of light on the molecular organization of the photosynthetic apparatus in Scenedesmus. Planta 173: 205–212.
Grünewald, K., Hagen, C. and Braune, W. 1997. Secondary carotenoid accumulation in flagellates of the green alga Haematococcus lacustris. Eur. J. Phycol. 32: 387–392.
Grünewald, K., Eckert, M., Hirschberg, J. and Hagen, C. 2000. Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiol. 122: 1261–1268.
Grünewald, K., Hirschberg, J. and Hagen, C. 2001. Ketocarotenoid biosynthesis outside of plastids in the unicellular green alga Haematococcus pluvialis. J. Biol. Chem. 276: 6023–6029.
Hirschberg, J. 2001. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant. Biol. 4: 210–218.
Johnson, E.A. and Schroeder, W.A. 1995. Microbial carotenoids. Adv. Biochem. Eng. Biotechnol. 53: 119–178.
Kobayashi, M., Kakizono, T., Nishio, N. and Nagai, S. 1992. Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 74: 61–63.
Kobayashi, M., Kakizono, T. and Nagai, S. 1993. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 59: 867–873.
Kobayashi, M., Kurimura, Y., Kakizono, T., Nishio, N. and Tsuji, Y. 1997. Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J. Ferment. Bioeng. 84: 94–97.
Kovacs, L., Wiessner, W., Kis, M., Nagy, F., Mende, D. and Demeter, S. 2000. Short-and long-term redox regulation of photosynthetic light energy distribution and photosystem stoichoimetry by acetate metabolism in the green alga, Chlamydobotrys stellata. Photosynth. Res. 65: 231–247.
Kujat, S.L. and Owttrim, G.W. 2000. Redox-regulated RNA helicase expression. Plant Physiol. 124: 703–714.
Lang, N.J. 1968. Electron microscopic studies of extraplastidic astaxanthin in Haematococcus. J. Phycol. 4: 12–19.
Linden, H. 1999. Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim. Biophys. Acta 1446: 203–212.
Linden, H., Misawa, N., Chamovitz, D., Pecker, I., Hirschberg, J. and Sandmann, G. 1991. Functional complementation in Escherichia coli of different phytoene desaturase genes and analysis of accumulated carotenes. Z. Naturforsch. [C] 46: 1045–1051.
Linden, H., Vioque, A. and Sandmann, G. 1993. Isolation of a carotenoid biosynthesis gene coding for ζ-carotene desaturase from Anabaena PCC 7120 by heterologous complementation. FEMS Microbiol. Lett. 106: 99–104.
Lorenz, R.T. and Cysewski, G.R. 2000. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 18: 160–167.
Misawa, N., Satomi, Y., Kondo, K., Yokoyama, A., Kajiwara, S., Saito, T., Ohtani, T. and Miki, W. 1995. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bact. 177: 6575–6584.
Pfannschmidt, T., Nilsson, A. and Allen, J.F. 1999. Photosynthetic control of chloroplast gene expression. Nature 397: 625–628.
Pfannschmidt, T., Allen, J.F. and Oelmuller, R. 2001a. Principles of redox control in photosynthesis gene expression. Physiol. Plant. 112: 1–9.
Pfannschmidt, T., Schütze, K., Brost, M. and Oelmüller, R. 2001b. A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J. Biol. Chem. 276: 36125–36130.
Scheibe, R. 1991. Redox-modulation of chloroplast enzymes. Plant Physiol. 96: 1–3.
Sokolowsky, V., Kaldenhoff, R., Ricci, M. and Russo, V.E.A. 1990. Fast and reliable mini-prep RNA extraction from Neurospora crassa. Fungal Genet. Newsl. 37: 41–43.
Steinbrenner, J. and Linden, H. 2000. Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin biosynthesis in the green alga Haematococcus pluvialis. Plant Physiol. 125: 810–817.
Sun, Z., Cunningham, F.X. and Gantt, E. 1998. Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular chlorophyte. Proc. Natl. Acad. Sci. USA 95: 11482–11488.
Trebst, A. 1980. Inhibitors in electron flow: tools for the functional and structural localization of carriers and energy conservation sites. Meth. Enzymol. 69: 675–715.
Tullberg, A., Alexciev, K., Pfannschmidt, T. and Allen, J.F. 2000. Photosynthetic electron flow regulates transcription of the psaB gene in pea (Pisum sativum L.) chloroplasts through the redox state of the plastoquinone pool. Plant Cell Physiol. 41: 1045–1054.
von Lintig, J., Welsch, R., Bonk, M., Giuliano, G., Batschauer, A. and Kleinig, H. 1997. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J. 12: 625–634.
Wild, A. 1979. Physiolgy of photosynthesis in higher plants. The adaptation to light intensity and light quality. Ber. Deut. Bot. Ges. 92: 341–364.
Yang, D.-H., Andersson, B., Aro, E.-M. and Ohad, I. 2001. The redox stat of the plastoquinone pool controls the level of the lightharvesting chlorophyll a/b binding protein complex II (LHC II) during photoacclimation. Photosynth. Res. 68: 163–174.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinbrenner, J., Linden, H. Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol 52, 343–356 (2003). https://doi.org/10.1023/A:1023948929665
Issue Date:
DOI: https://doi.org/10.1023/A:1023948929665