Abstract
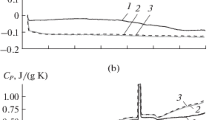
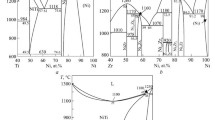
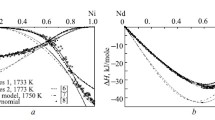
The vapor composition over and thermodynamic properties of crystalline nickel–phosphorus alloys (26–32.5 at. % P) are studied by Knudsen cell mass spectrometry between 971 and 1440 K. Mass spectra of the saturated vapor in the Ni–P system indicate the presence of the Ni+, P+ 2, and P+ ions. The P concentration in the vapor phase does not exceed 1% of the Р2 concentration. The experimental data are used to evaluate the complete set of thermodynamic functions of nickel phosphides. Based on the data for the low-temperature (α, γ) and high-temperature (β, δ) forms of Ni5P2 and Ni12P5 , the thermodynamic characteristics of the corresponding polymorphic transformations are evaluated. The results obtained for a large number of compositions under different experimental conditions (different effusion-cell and coating materials and effusion-orifice diameters) coincide to within the experimental error and are in good agreement with earlier reported phase-equilibrium data and enthalpies of formation, attesting to the high accuracy and reliability of the calculated thermodynamic functions.
Similar content being viewed by others
REFERENCES
Wachtel, E., Bakonyi, I., Wllman, N., et al., Magnetic Susceptibility and DSC Study of the Crystallization of Melt-Quenched Ni-P Amorphous Alloys, Mater.Sci.Eng., A, 1991, vol.133, pp.196-199.
Weibke, F.and Schrag, R., Die Bildungswarmen der Niederen Phosphide einiger Schwermetalle, Z.Elektrochem., 1941, vol.47, no.3, pp.222-238.
Myers, C.E.and Conti, T.G., Vaporization Behavior, Phase Equilibria, and Thermodynamic Stabilities of Nickel Phosphides, J.Electrochem.Soc., 1985, vol.132, no.2, pp.454-457.
Boone, S.and Kleppa, O.J., Determination of the Standard Enthalpy of Formation of Ni 2.55 P by High-Temperature Drop Calorimetry, J.Chem.Thermodyn., 1991, vol.23, pp.781-790.
Viksman, G.Sh.and Gordienko, S.P., High-Temperature Behavior of Nickel Phosphide in Vacuum and Its Thermodynamic Characteristics, Poroshk.Metall. (Kiev), 1992, no.12, pp.70-72.
Zaitsev, A.I., Zemchenko, M.A., and Mogutnov, B.M., Vapor Pressure of Iron, Zh.Fiz.Khim., 1990, vol.64, no.12, p.3377.
Zaitsev, A.I., Shelkova, N.E., Litvina, A.D., et al., Vaporization Behavior of Liquid Iron-Copper Alloys, Teplofiz.Vys.Temp., 2001, vol.39, no.3, pp.416-423.
Zaitsev, A.I., Zemchenko, M.A., and Mogutnov, B.M., Thermodynamic Properties of Manganese Silicides, Zh.Fiz.Khim., 1989, vol.63, no.6, pp.1451-1458.
Zaitsev, A.I., Dobrokhotova, Zh.V., Litvina, A.D., and Mogutnov, B.M., Thermodynamic Properties and Phase Equilibrium in the Fe-P System, J.Chem.Soc., Faraday Trans., 1995, vol.93, no.4, pp.703-712.
Zaitsev, A.I., Korolyov, N.V., and Mogutnov, B.M., Thermodynamic Properties of {x Ca + (1 - x )P}, J.Chem.Thermodyn., 1991, vol.23, pp.11-23.
Zaitsev, A.I., Korolyov, N.V., and Mogutnov, B.M., The Vapour Pressures and the Heats of Sublimation of CaF 2 and SrF 2, High Temp.Sci., 1990, vol.28, p.341.
Sidorov, L.N., Korobov, M.V., and Zhuravleva, L.V., Mass-spektral'nye termodinamicheskie issledovaniya (Mass Spectrometric Thermodynamic Studies), Moscow: Mosk.Gos.Univ., 1985.
Mann, J.B., Recent Development in Mass-Spectroscopy, Proc.Int.Conf.on Mass Spectroscopy, Tokyo, 1970, p.814.
Zaitsev, A.I.and Zaitseva, N.E., High-Temperature Vapor Pressure of Nickel, Teplofiz.Vys.Temp., 2002, vol.40, no.2, pp.225-230.
Karakaya, I.and Thompson, W.T., The Ag-P (Argentum- Phosphorus) System, Bull.Alloy Phase Diagrams, 1988, vol.9, no.3, p.232.
Dinsdale, A.T., SGTE Data for Pure Elements, CALPHAD: Comput.Coupling Phase Diagrams Thermochem., 1991, vol.15, no.4, p.317.
Lee, K.J.and Nash, P., Phase Diagrams of Binary Nickel Alloys, Nash, P., Ed., Materials Park: ASM International, 1991, pp.235-246.
Shim., J.-H., Chung, H.-J., and Lee, D.N., Calculation of Phase Equilibria and Evaluation of Glass-Forming Ability of Ni-P Alloys, J.Alloys Compd., 1999, vol.282, pp.175-181.
Yurko, L.M., Svirid, A.A., and Muchnik, S.V., Phase Equilibria in the Systems Nickel-Phosphorus and Nickel-Phosphorus-Carbon, Poroshk.Metall. (Kiev), 1986, no.9, pp.78-83.
JANAF Thermochemical Tables, 2nd ed., J.Phys.Chem.Ref.Data, 1985, vol.14.
Lu, K., Luck, R., and Predel, B., Investigation of the Heat Capacities of Ni-20 at.% P in Different States, Z.Metallkd., 1993, vol.84, no.11, pp.740-743.
Lu, K., Wang, J.T., and Wei, W.D., Thermal Expansion and Specific Heat Capacity of Nanocrystalline Ni-P Alloy, Scr.Metall.Mater., 1991, vol.25, pp.619-623.
Jacobs, R.B., Chem.Phys., 1937, vol.5, p.945.
O'Hare, P.A.G.and Hubbard, W.N., J.Chem.Soc., Faraday Trans., 1966, vol.62, p.2709.
Zaitsev, A.I., Zemchenko, M.A., and Mogutnov, B.M., Thermodynamic Properties of Mn-P Alloys, Z.Metallkd., 1993, vol.84, no.3, pp.178-184.
Gel'd, P.V.and Sidorenko, F.A., Silitsidy perekhodnykh metallov chetvertogo perioda (3 d Transition Metal Silicides), Moscow: Metallurgiya, 1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zaitsev, A.I., Zaitseva, N.E. & Shakhpazov, E.K. Thermodynamic Properties of Intermediate Ni–P Phases (26–32.5 at. % P) between 971 and 1440 K. Inorganic Materials 39, 427–432 (2003). https://doi.org/10.1023/A:1023643721556
Issue Date:
DOI: https://doi.org/10.1023/A:1023643721556