Abstract
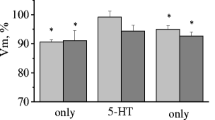
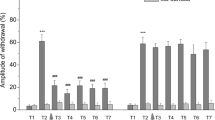
The nature of the effects of opioid peptides on the properties of electrogenic membranes and the responses of defensive behavior command neurons LPl1 and RPl1, evoked by sensory stimuli of different modalities and application sites was studied in semi-intact preparations from common snails. Application of met-enkephalin (10 μM) to the snail CNS produced increases in membrane excitability along with facilitation of responses to application of dilute quinine solution to the animal's head and depression of responses to tactile stimulation of the head. Met-enkephalin (0.1 μM) produced only depression of responses to tactile stimulation of the head. Application of leu-enkephalin (10 μM) was accompanied by depression of responses to tactile stimulation of the head. Membrane excitability and responses to chemical sensory stimulation during application showed no change during application of this peptide. These effects of both peptides appeared 10–20 min from the start of application and lasted 15–30 min after washing was started. In addition, facilitation of the responses of neurons to chemical sensory stimulation was seen 30–50 min after the start of leu-enkephalin application. The responses of neurons to tactile stimulation of the snail's foot were not altered by application of peptides. The neuronal effects of peptides were suppressed by simultaneous application of naloxone (50 μM). Thus, we observed the selective action of opioid peptides on the synaptic plasticity of neurons LPl1 and RPl1, both in relation to the location of sensory stimulation and in relation to sensory modality.
Similar content being viewed by others
REFERENCES
V. E. D'yakonova and D. A. Sakharov, “The neurotransmitter basis of mollusk behavior: control by the selection between orientational and defensive responses to presentation of an unfamiliar object,” Zh. Vyssh. Nerv. Deyat., 44, No. 3, 526–531 (1994).
T. L. D'yakonova, “The effects of enkephalins on the somatic membranes of identified neurons in the common snail,” in: Simple Nervous Systems. Proceedings of the II All-Union Conference [in Russian], Nauka, Leningrad (1988), pp. 93–96.
T. L. D'yakonova, “Interaction between opioid peptides and monoamines in the mechanisms controlling the respiratory behavior of a pulmonate mollusk: analysis of isolated neurons,” Dokl. Akad. Nauk SSSR, 308, No. 5, 1264–1269 (1989).
T. L. D'yakonova and G. G. Arakelov, “The monosynaptic connection: the modulating effects of opioid peptides on the plasticity people presynaptic neurons and identified synapses,” Zh. Vyssh. Nerv. Deyat., 41, No. 4, 788–795 (1991).
O. A. Maksimova and P. M. Balaban, Neuronal Mechanisms of Behavioral Plasticity [in Russian], Nauka, Moscow (1983).
V. P. Nikitina, “A transient stage of long-term synaptic facilitation in defensive behavior command neurons in sensitized snails,” Ros. Fiziol. Zh. im. I. M. Sechenova, 85, No. 1, 36–47 (1999).
V. P. Nikitin, S. A. Kozyrev, and A. V. Shevelkin, “Antagonists of NMDA glutamate receptors selectively influence synaptic mechanisms of nociceptive sensitization in snails,” Zh. Vyssh. Nerv. Deyat., 50, No. 3, 601–610 (2000).
T. P. Norekyan and D. A. Sakharov, “Mechanoreception in the pteropod mollusk Clione limacina; tactile inputs are blocked by opiate antagonists,” Sensor. Sistemy, 5, No. 3, 5–11 (1991).
A. S. Pivovarov, “Differently directed modulation of cholinoreceptor plasticity of RPa3 and LPa3 neurons by opiate mu and kappa agonists in the common snail,” Zh. Vyssh. Nerv. Deyat., 43, No. 4, 826–836 (1993).
R. N. Skryma, “Modulation of the functioning of muscarinic and nicotinic cholinoreceptors of dialyzed snail neurons by opiate peptides and morphine,” Neirofiziologiya, 14, No. 6, 654–656 (1982).
E. I. Solntseva, “The role of cAMP in the electrophysiological effects of morphine and enkephalins,” Zh. Vyssh. Nerv. Deyat., 43, No. 5, 946–952 (1993).
Kh. P. Tiras, A. Yu. Rubina, Yu. V. Miloserdov, and V. A. Tishchenko, “A morphogen of Hydra as a possible endogenous stimulator of regeneration of planarians,” in: Morphogenetically Active Substances [in Russian], ONTI NtsBI of the Academy of Sciences of the USSR, Pushchino (1990), pp. 134–135.
A. V. Shevelkin, “Facilitation of defensive responses during the period of food consumption in the common snail: the role of glucose and gastrin/cholecystokinin-like peptide,” Zh. Vyssh. Nerv. Deyat., 42, No. 6, 1235–1249 (1992).
I. M. Sheiman, Kh. P. Tiras, V. A. Vinogradov, and I. A. Efimov, “The enkephalin analog dalargin accelerates regeneration of the cephalic end of the body of planarians,” Dokl. Akad. Nauk SSSR, 284, No. 2, 481–483 (1985).
K. Elekes, G. B. Stefano, and D. O. Carpenter, “Enkephalin-like immunoreactive neurons in the central nervous system of gastropods (Helix pomatia, Lymnaea stagnalis, Aplysia californica). A comparative immunocytochemical study,” Cell Tiss. Res., 272, No. 2, 329–341 (1993).
R. Gutierrez and M. Asai, “IR-Met and IR-Leu-enkephalin content in the perioesophageal ganglia of Helix aspersa seasonal variations,” Comp. Biochem. Physiol. C100, No. 3, 609–613 (1991).
L. M. Harrison, A. J. Kastin, J. Weber, et al., “The opiate system in invertebrates,” Peptides, 15, No. 6, 1309–1321 (1994).
K. Lukowiak, J. A. Thornhill, and J. Edstrom, “Methionineenkephalin increases CNS suppressive control exerted over gill reflex behaviours and associated neural activity in Aplysia californica,” Reg. Peptides, 3, No. 2, 303–312 (1982).
O. A. Maksimova, N. I. Bravarenko, and P. M. Balaban, “Two modulatory inputs exert reciprocal reinforcing effects on synaptic input of premotor interneurons for withdrawal in terrestrial snails,” Learn. Mem., 6, No. 1, 168–176 (1999).
G. Martin, Z. Nie, and G. R. Siggins, “mu-Opioid receptors modulate NMDA receptor-mediated responses in nucleus accumbens neurons,” J. Neurosci., 17, No. 1, 11–22 (1997).
G. A. Olson, R. D. Olson, and A. J. Kastin, “Endogenous opiates: 1992,” Peptides, 14, No. 6, 1339–1358 (1993).
E. Perl, E. Shuffman, A. Vas, S. Luger, and J. E. Steiner, “Taste-and odor-reactivity in heroin addicts,” Isr. J. Psychiatry Relat. Sci., 34, No. 4, 290–299 (1997).
K. S. Rozsa and E. J. Solntseva, “Modulation of cholinergic transmission by opiate peptide and FMRFamide on identified neurons of Helix pomatia L. (Gastropoda, Mollusca),” Acta Physiol Hung., 67, No. 4, 429–433 (1986).
K. I. Rusin and H. C. Moises, “mu-Opioid receptor activation reduces multiple components of high-threshold calcium current in rat sensory neurons,” J. Neurosci., 15, No. 6, 4315–4327 (1995).
E. Salo and J. Baguna, “Stimulation of cellular proliferation and differentiation in the intact and regenerating planarian Dugesia (G) tigrina by the neuropeptide substance P,” J. Exper. Zool., 277, No. 1, 129–135 (1986).
R. L. Seltner, B. Rohrer, V. Grant, and W. K. Stell, “Endogenous opiates in the chick retina and their role in form-deprivation myopia,” Vis. Neurosci., 14, No. 5, 801–809 (1997).
A. W. Thomas, M. Kavaliers, F. S. Prato, and K. P. Ossenkopp, “Pulsed magnetic field induced ‘analgesia’ in the land snail, Cepaea nemoralis, and the effects of mu, delta and kappa opioid receptor agonists/antagonists,” Peptides, 18, No. 5, 703–709 (1997).
S. Willenbring and C. W. Stevens, “Spinal mu, delta and kappa opioids alter chemical, mechanical and thermal sensitivities in amphibians,” Life Sci., 61, No. 22, 2167–2176 (1997).
C. W. Xie and D. V. Lewis, “Involvement of cAMP-dependent protein kinase in mu-opioid modulation of NMDA-mediated synaptic currents,” J. Neurophysiol., 78, No. 2, 759–766 (1997).
L. Xin, E. B. Geller, and M. W. Adler, “Body temperature and analgesic effects of selective mu and kappa opioid receptor microdialyzed into rat brain,” J. Pharmacol. Exp. Ther., 28, No. 1, 499–507 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nikitin, V.P., Kozyrev, S.A. & Shevelkin, A.V. The Selective Action of Opioid Peptides on Excitability and the Various Sensory Inputs of Defensive Behavior Command Neurons LPl1 and RPl1 of the Common Snail. Neurosci Behav Physiol 33, 447–453 (2003). https://doi.org/10.1023/A:1023407116143
Issue Date:
DOI: https://doi.org/10.1023/A:1023407116143