Abstract
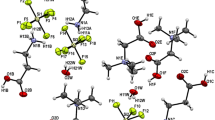
Orthoperiodic and orthotelluric acids, their salts MIO6H4 (M = Li, Rb, Cs) and CsH5TeO6, and dimers of the salt · acid type are calculated within density functional theory B3LYP and basis set LanL2DZ complemented by the polarizationd,p-functions. According to calculations, the salt · acid dimerization is energetically favorable for compounds MIO6H4 · H5IO6 (M = Rb, Cs) and CsIO6H4 · H6TeO6. The dimerization energy is equal to 138–146 kJ mol–1. With relatively small activation energies equal to 4 kJ mol–1 (M = Li) and 11 kJ mol–1 (M = Rb, Cs), possible is rotation of octahedron IO6 relative to the M atom in monomers of salt molecules. The proton transfer along an octahedron occurs with activation energies of 63–84 kJ mol–1. The activation energy for the proton transfer between neighboring octahedrons of the type salt · acid → acid · salt equals 8–17 kJ mol–1. Quantum-chemical calculations nicely conform to x-ray diffraction and electrochemical data.
Similar content being viewed by others
References
Haile, S.M., Bousem, D.A., Chistolm, C.R.I., and Merle, R.B., Nature (London), 2001, vol. 410, p. 910.
Norby, T., Nature (London), 2001, vol. 410, p. 877.
Baranov, A.I., Shuvalov, L.A., and Shchagina, M.M., Pis'ma Zh. Eksp. Teor. Fiz., 1982, vol. 36, p. 459.
Pawlowski, A., Pawlaczyk, Cz., and Hiltezer, B., Solid State Ionics, 1990, vol. 44, p. 910.
Chistolm, C.R.I. and Haile, S.M., Solid State Ionics, vol. 136-137, p. 229.
Shilov, G.V., Dobrovol'skii, Yu.A., Chernyak, A.V., et al., Koord. Khim., 2001, vol. 27, p. 834.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al., Gaussian 94, Pittsburgh (PA): Gaussian Inc., 1995, Revision D.1.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al., Gaussian 98, Pittsburgh (PA): Gaussian Inc., 1998, Revision A.
Jursic, B.S., J. Mol. Struct., 1998, vol. 427, p. 137.
Jursic, B.S., J. Mol. Struct., 1998, vol. 427, p. 157.
Rodriquez, C.F., Cunje, A., and Hopkinson, A.C., J. Mol. Struct., 1998, vol. 430, p. 149.
Feikema, Y.D., Acta Crist., 1966, vol. 20, p. 765.
Feikema, Y.D. and Vos, A., Acta Cristallogr., 1966, vol. 20, p. 769.
Kalman, A. and Cruickshank, D.W., Acta Crystallogr., Sect. B: Struct. Sci., 1970, vol. 26, p. 1782.
Kellersohn, T., Acta Crystallogr., 1991, vol. 47, p. 1133.
Abrahams, S.C. and Bernstein, J.L., J. Chem. Phys., 1978, vol. 69, p. 4234.
Ferrari, A., Braibanti, A., and Tiripicchio, A., Acta Crystallogr., 1965, vol. 19, p. 629.
Averbuch-Pouchot, M.T., J. Solid State Chem., 1983, vol. 49, p. 368.
Zilber, R., Durif, A., and Averbuch-Pouchot, M.T., Acta Crystallogr., Sect. B: Struct. Sci., 1981, vol. 37, p. 650.
Dammak, M., Khemakhem, H., Mhiri, T., et al., J. Alloys Compd., 1998, vol. 280, p. 107.
Zilber, R., Tordjman, I., and Guitel, J.C., Acta Crystallogr., Sect. B: Struct. Sci., 1980, vol. 36, p. 2741.
Zilber, R., Durif, A., and Averbuch-Pouchot, M.T., Acta Crystallogr., Sect. B: Struct. Sci., 1980, vol. 36, p. 2743.
Berry, R.S., J. Chem. Phys., 1960, vol. 32, p. 933.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zyubina, T.S., Shilov, G.V., Dobrovol'skii, Y.A. et al. Modeling the Proton Transport in Orthoperiodic and Orthotelluric Acids and Their Salts. Russian Journal of Electrochemistry 39, 376–385 (2003). https://doi.org/10.1023/A:1023357922020
Issue Date:
DOI: https://doi.org/10.1023/A:1023357922020