Abstract
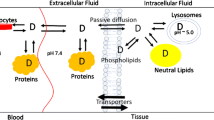
The tissue-to-unbound plasma distribution coefficients (Kpus) of 14 rat tissues after iv administration of nine 5-n-alkyl-5-ethyl barbituric acids, determined in a previous study, were used to identify a model of the relationship between tissue distribution and lipophilicity of the homologs, expressed in terms of their octanol to water partition ratio, P. Based on mechanistic considerations and assumptions, the parameter model was expressed asKpuτ= fW,τ[l + aτ(nPl,τ)Pbτ], where fW,τ is the tissue water content, (nPl,τ ) is the binding capacity of the tissue, n is the number of the binding sites, aτ and bτ are the parameters of the relationship Kaτ = aτPbτ and Kaτ is the binding association constant of each tissue. The parameter model was linearized and fitted to the predetermined Kpu values, yielding correlation coefficients ranging between .940 and .997. The predictive performance of the parameter model was evaluated using a leave-one-out procedure with subsequent computation of the mean prediction error (ME = measurement of the prediction bias) and the square root of the mean squared prediction error (RMSE = measurement of the prediction accuracy). The ME varied between −22.48 and 61.14%, indicating a slight tendency for overpredicting. The RMSE was between 24.73 and 102% for the individual tissues across the different homologs; and between 28.33 and 85.2% for the individual homologs across the different tissues. The apparently high Kpu prediction errors, when translated through the low sensitivity of the barbiturate whole-body physiologically based pharmacokinetic model, established previously, leads to predicted tissue concentration–time profiles within 5 to 20% of the original ones. Therefore, it is concluded, that the identified mechanistically based model is a good predictor of the tissue-to-unbound Kpus in the rat tissues.
Similar content being viewed by others
REFERENCES
L. W. Frick, K. K. Adkinson, K. J. Wells-Knecht, P. Woolland, and D. M. Highton. Cassette dosing: rapid in vivo assessment of pharmacokinetics. PSTT 1:12–18 (1998).
J. Berman, K. Halm, K. Adkinson, and J. Shaffer. Simultaneous pharmacokinetic screening of a mixture of compounds in the dig using API LC/MS/MS analysis for increased throughput. J. Med. Chem. 40:827–829 (1997).
J. K. Seydel and K-J. Schaper. Quantitative structure-pharmacokinetic relationships and drug design. In M. Rowland and G. Tucker (eds.), Pharmacokinetics: Theory and Methodology. International Encyclopedia of Pharmacology and Therapeutics, Section 122, Pergamon, Oxford, 1986, pp. 311–368.
G. E. Blakey, I. A. Nestorov, P. A. Arundel, L. J. Aarons, and M. Rowland. Quantitative structure-pharmacokinetics relationships: I. Development of a whole-body physiologically based model to characterize changes in pharmacokinetics across a homologous series of barbiturates in the rat. J. Pharmacokin. Biopharm. 25:277–312 (1997).
S. Toon and M. Rowland. Structure-activity relationships among the barbiturates in the rat. J. Pharmacol. Exp. Ther. 225:752–763 (1983).
M. Rowland and T. N. Tozer. Clinical Pharmacokinetics: Concepts and Applications, 3rd ed., Williams and Wilkins, Philadelphia, 1995, p. 600.
P. H. Hinderling. Red blood cells: A neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol. Rev. 49:279–295 (1997).
I. A. Nestorov, L. J. Aarons, P. A. Arundel, and M. Rowland. Lumping of whole-body physiologically based pharmacokinetic models. J. Pharmacokin. Biopharm. 26:23–46 (1998).
K. Yokogawa, E. Nakashima, J. Ishizaki, H. Maeda, T. Nagano, and F. Ichimura. Relationships in the structure-tissue distribution of basic drugs in the rabbit. Pharm. Res. 7:691–696 (1990).
J. Ishizaki, K. Yokogawa, E. Nakashima, and F. Ichimura. Relationships between the hepatic intrinsic clearance or blood cell-plasma partition coefficient in the rabbit and the lipophilicity of basic drugs. J. Pharm. Pharmacol. 49:768–772 (1997).
J. Ishizaki, K. Yokogawa, E. Nakashima, and F. Ichimura. Prediction of changes in the clinical pharmacokinetics of basic drugs on the basis of octanol-water partition coefficients. J. Pharm. Pharmacol. 49:762–767 (1997).
R. F. Reinoso, B. A. Telfer, and M. Rowland. Tissue water contents in rats measured by desiccation. J. Pharmacol. Toxicol. Meth. 38:87–92 (1997).
L. B. Sheiner and S. Beal. Some suggestions for measuring predictive performance. J. Pharmacokin. Biopharm. 9:503–512 (1981).
ACSL Reference Manual, Edition 11. MGA Software, Concord MA 01742, USA, 1995.
A. Leo and C. Hansch. Linear free-energy relationships between partitioning solvent systems. J. Org. Chem. 36:1539–1544 (1971).
K. B. Bischoff and R. L. Dedrick. Thiopental pharmacokinetics. J. Pharm. Sci. 57:1346–1351 (1968).
P. Barton, A. M. Davis, D. J. McCarthy, and P. J. H. Webborn. Drug-Phospholipid Interactions: 2. Predicting the sites of drug distribution using n-octanol/water and membrane/water distribution coefficient. J. Pharm. Sci. 86:1034–1039 (1997).
T. D. Yih and J. M. Van Rossum. Pharmacokinetics of some homologous series of barbiturates in the intact rat and in the isolated perfused rat liver. J. Pharmacol. Exp. Ther. 203:182–763 (1977).
J. M. Mayer, S. D. Hall, and M. Rowland. Relationship between lipophilicity and tubular reabsorption for a series of 5–alkyl-5–ethyl barbituric acids in the isolated perfused rat kidney preparation. J. Pharm. Sci. 77:359–364 (1988).
R. J. Prankerd and R. H. McKeown. Physicochemical properties of barbituric acid derivatives: 2. Partition coefficients of 5,5–disubstituted barbituric acids at 25°C. Int. J. Pharm. 83:25–37 (1992).
R. Pinal and S. H. Yalkowsky. Solubility and partitioning VII: Solubility of barbiturates in water. J. Pharm. Sci. 76:75–85 (1987).
S. H. Steiner, M. J. Moor, and M. H. Bickel. Kinetics of distribution and adipose tissue storage as a function of lipophilicity and chemical structure: 1. Barbiturates. Drug Metab. Dispos. 19:8–14 (1991).
B. B. Brodie, E. Bernstein, and L. C. Mark. The role of body fat in limiting the duration of action of thiopental. J. Pharm. Pharmacol. 105:421–426 (1952).
J. M. Gallo, F. C. Lam, and D. G. Perrier. Area method for the estimation of partition coefficients for physiological pharmacokinetic models. J. Pharmacokinet. Biopharm. 15:271–280 (1987).
D. Verotta, L. B. Sheiner, W. F. Ebling, and D. Stanski. A semiparametric approach to physiological flow models. J. Pharmacokinet. Biopharm. 17:463–491 (1989).
P. Poulin and K. Krishnan. A tissue composition-based algorithm for predicting tissue:air partition coefficients of organic chemicals. Toxicol. Appl. Pharmacol. 136:126–130 (1996).
M. Pelekis, P. Poulin, and K. Krishnan. An approach for incorporating tissue composition data into physiologically based pharmacokinetic models. Toxicol. Ind. Health 11:511–522 (1995).
F. M. Parham, M. C. Kohn, H. B. Matthews, C. DeRosa, and C. J. Portier. Using structural information to create physiologically based pharmacokinetic models for all polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 144:340–347 (1997).
KOWWIN. Octanol-Water Partition Coefficient Program. User's Guide, Syracuse Research Corporation, Merill Lane, Syracuse, NY, 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nestorov, I., Aarons, L. & Rowland, M. Quantitative Structure–Pharmacokinetics Relationships: II. A Mechanistically Based Model to Evaluate the Relationship Between Tissue Distribution Parameters and Compound Lipophilicity. J Pharmacokinet Pharmacodyn 26, 521–545 (1998). https://doi.org/10.1023/A:1023221116200
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1023221116200