Abstract
Purpose of Review
Prior to human studies, knowledge of drug disposition in the body is useful to inform decisions on drug safety and efficacy, first in human dosing, and dosing regimen design. It is therefore of interest to develop predictive models for primary pharmacokinetic parameters, clearance, and volume of distribution. The volume of distribution of a drug is determined by the physiological properties of the body and physiochemical properties of the drug, and is used to determine secondary parameters, including the half-life. The purpose of this review is to provide an overview of current methods for the prediction of volume of distribution of drugs, discuss a comparison between the methods, and identify deficiencies in current predictive methods for future improvement.
Recent Findings
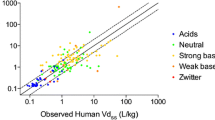
Several volumes of distribution prediction methods are discussed, including preclinical extrapolation, physiological methods, tissue composition-based models to predict tissue:plasma partition coefficients, and quantitative structure-activity relationships. Key factors that impact the prediction of volume of distribution, such as permeability, transport, and accuracy of experimental inputs, are discussed. A comparison of current methods indicates that in general, all methods predict drug volume of distribution with an absolute average fold error of 2-fold. Currently, the use of composition-based PBPK models is preferred to models requiring in vivo input.
Summary
Composition-based models perfusion-limited PBPK models are commonly used at present for prediction of tissue:plasma partition coefficients and volume of distribution, respectively. A better mechanistic understanding of important drug distribution processes will result in improvements in all modeling approaches.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rowland M, Tozer T. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. Fourth ed. 2011.
Gabrielsson J, Weiner D. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications. Fourth ed. 2010.
Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1):46–58.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76. https://doi.org/10.1002/jps.20322.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57. https://doi.org/10.1002/jps.20502.
Hardman JG, Limbird LE. Goodman and Gilman's the pharmacological basis of therapeutics 10th edition. New York: McGraw-Hill; 2001.
Peters SA. Physiologically-based pharmacokinetic modeling and simulations. Hoboken, NJ: Wiley; 2012.
Cole S, Bagal S, El-Kattan A, Fenner K, Hay T, Kempshall S, et al. Full efficacy with no CNS side-effects: unachievable panacea or reality? DMPK considerations in design of drugs with limited brain penetration. Xenobiotica. 2012;42(1):11–27. https://doi.org/10.3109/00498254.2011.617847.
Shitara Y, Maeda K, Ikejiri K, Yoshida K, Horie T, Sugiyama Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos. 2013;34(1):45–78. https://doi.org/10.1002/bdd.1823.
Boxenbaum H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 1982;10(2):201–27. https://doi.org/10.1007/BF01062336.
Freitas AA, Limbu K, Ghafourian T. Predicting volume of distribution with decision tree-based regression methods using predicted tissue:plasma partition coefficients. J Cheminformatics. 2015;7:17. https://doi.org/10.1186/s13321-015-0054-x.
Mahmood I. Theoretical versus empirical allometry: facts behind theories and application to pharmacokinetics. J Pharm Sci. 2010;99(7):2927–33. https://doi.org/10.1002/jps.22073.
Colclough N, Ruston L, Wood JM, MacFaul PA. Species differences in drug plasma protein binding. Med Chem Commun. 2014;5:963–7.
Sugita O, Sawada Y, Sugiyama Y, Hanano M, Iga T. Effect of sulfaphenazole on tolbutamide distribution in rabbits - analysis of interspecies differences in tissue distribution of tolbutamide. J Pharm Sci. 1984;73(5):631–4. https://doi.org/10.1002/jps.2600730513.
Sawada Y, Hanano M, Sugiyama Y, Harashima H, Iga T. Prediction of the volumes of distribution of basic drugs in humans based on data from animals. J Pharmacokinet Biopharm. 1984;12(6):587–96. https://doi.org/10.1007/bf01059554.
Jones R, Jones HM, Rowland M, Gibson CR, Yates JWT, Chien JY, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 2: comparative assessment of prediction methods of human volume of distribution. J Pharm Sci. 2011;100(10):4074–89. https://doi.org/10.1002/jps.22553.
Gillette JR. Factors affecting drug metabolism. Ann N Y Acad Sci. 1971;179:43–66.
Gibaldi M, McNamara PJ. Apparent volumes of distribution and drug binding to plasma proteins and tissues. Eur J Clin Pharmacol. 1978;13(5):373–80.
Wilkinson GR, Shand DG. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18(4):377–90.
Oie S, Tozer TN. Effect of altered plasma-protein binding on apparent volume of distribution. J Pharm Sci. 1979;68(9):1203–5. https://doi.org/10.1002/jps.2600680948.
Lombardo F, Obach RS, Shalaeva MY, Gao F. Prediction of volume of distribution values in humans for neutral and basic drugs using physicochemical measurements and plasma protein binding data. J Med Chem. 2002;45(13):2867–76. https://doi.org/10.1021/jm0200409.
Lombardo F, Obach RS, Shalaeva MY, Gao F. Prediction of human volume of distribution values for neutral and basic drugs. 2. Extended data set and leave-class-out statistics. J Med Chem. 2004;47(5):1242–50. https://doi.org/10.1021/jm030408h.
•• Korzekwa K, Nagar S. Drug Distribution Part 2. Predicting volume of distribution from plasma protein binding and membrane partitioning. Pharm Res. 2017;34(3):544–51. https://doi.org/10.1007/s11095-016-2086-y This article describes a new method for the prediction of the Vss ,which utilizes partitioning into microsomes to represent phospholipid partitioning in a physiological-based Vss equation. This study also looked at other tissue interactions which may be important for describing the distribution of a drug.
Arundel P. A multi-compartmental model generally applicable to physiologically-based pharmacokinetics. IFAC Proceedings Volumes. 1997;30(2):129–33. https://doi.org/10.1016/S1474-6670(17)44557-5.
Jansson R, Bredberg U, Ashton M. Prediction of drug tissue to plasma concentration ratios using a measured volume of distribution in combination with lipophilicity. J Pharm Sci. 2008;97(6):2324–39. https://doi.org/10.1002/jps.21130.
Bjorkman S. Prediction of the volume of distribution of a drug: which tissue-plasma partition coefficients are needed? J Pharm Pharmacol. 2002;54(9):1237–45. https://doi.org/10.1211/002235702320402080.
Poulin P, Theil F-P. A priori prediction of tissue:plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J Pharm Sci. 2000;89(1):16–35. https://doi.org/10.1002/(sici)1520-6017(200001)89:1<16::aid-jps3>3.0.co;2-e.
Poulin P, Schoenlein K, Theil FP. Prediction of adipose tissue: plasma partition coefficients for structurally unrelated drugs. J Pharm Sci. 2001;90(4):436–47. https://doi.org/10.1002/1520-6017(200104)90:4<436::aid-jps1002>3.0.co;2-p.
Poulin P, Krishnan K. A biologically-based algorithm for predicting human tissue-blood partition coefficients of organic chemicals. Hum Exp Toxicol. 1995;14(3):273–80. https://doi.org/10.1177/096032719501400307.
Poulin P, Theil F-P. Development of a novel method for predicting human volume of distribution at steady-state of basic drugs and comparative assessment with existing methods. J Pharm Sci. 2009;98(12):4941–61. https://doi.org/10.1002/jps.21759.
Graham H, Walker M, Jones O, Yates J, Galetin A, Aarons L. Comparison of in-vivo and in-silico methods used for prediction of tissue: plasma partition coefficients in rat. J Pharm Pharmacol. 2012;64(3):383–96. https://doi.org/10.1111/j.2042-7158.2011.01429.x.
Berezhkovskiy LM. Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J Pharm Sci. 2004;93(6):1628–40. https://doi.org/10.1002/jps.20073.
Berry LM, Roberts J, Be X, Zhao Z, Lin MHJ. Prediction of Vss from in vitro tissue-binding studies. Drug Metab Dispos. 2010;38(1):115–21. https://doi.org/10.1124/dmd.109.029629.
Clausen J, Bickel MH. Prediction of drug distribution in distribution dialysis and in vivo from binding to tissues and blood. J Pharm Sci. 1993;82(4):345–9. https://doi.org/10.1002/jps.2600820402.
Poulin P, Ekins S, Theil F-P. A hybrid approach to advancing quantitative prediction of tissue distribution of basic drugs in human. Toxicol Appl Pharmacol. 2011;250(2):194–212. https://doi.org/10.1016/j.taap.2010.10.014.
Yun YE, Edginton AN. Correlation-based prediction of tissue-to-plasma partition coefficients using readily available input parameters. Xenobiotica. 2013;43(10):839–52. https://doi.org/10.3109/00498254.2013.770182.
Yun YE, Cotton CA, Edginton AN. Development of a decision tree to classify the most accurate tissue-specific tissue to plasma partition coefficient algorithm for a given compound. J Pharmacokinet Pharmacodyn. 2014;41(1):1–14. https://doi.org/10.1007/s10928-013-9342-0.
Schmitt W. General approach for the calculation of tissue to plasma partition coefficients. Toxicol In Vitro. 2008;22(2):457–67. https://doi.org/10.1016/j.tiv.2007.09.010.
Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci. 2002;91(1):129–56. https://doi.org/10.1002/jps.10005.
•• Korzekwa K, Nagar S. On the nature of physiologically-based pharmacokinetic models –a priori or a posteriori? Mechanistic or empirical? Pharm Res. 2017;34(3):529–34. https://doi.org/10.1007/s11095-016-2089-8 This article provides a commentary on the current assumptions and methods used in physiologically-based pharmacokinetic models.
Hinderling PH. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279–95.
Ye M, Nagar S, Korzekwa K. A physiologically based pharmacokinetic model to predict the pharmacokinetics of highly protein-bound drugs and the impact of errors in plasma protein binding. Biopharm Drug Dispos. 2016;37(3):123–41. https://doi.org/10.1002/bdd.1996.
Ghafourian T, Barzegar-Jalali M, Hakimiha N, Cronin MTD. Quantitative structure-pharmacokinetic relationship modelling: apparent volume of distribution. J Pharm Pharmacol. 2004;56(3):339–50. https://doi.org/10.1211/0022357022890.
Lombardo F, Obach RS, DiCapua FM, Bakken GA, Lu J, Potter DM, et al. Hybrid mixture discriminant analysis-random forest computational model for the prediction of volume of distribution of drugs in human. J Med Chem. 2006;49(7):2262–7. https://doi.org/10.1021/jm050200r.
Zhivkova Z, Doytchinova I. Prediction of steady-state volume of distribution of acidic drugs by quantitative structure-pharmacokinetics relationships. J Pharm Sci. 2012;101(3):1253–66. https://doi.org/10.1002/jps.22819.
Korzekwa KR, Nagar S, Tucker J, Weiskircher EA, Bhoopathy S, Hidalgo IJ. Models to predict unbound intracellular drug concentrations in the presence of transporters. Drug Metab Dispos. 2012;40(5):865–76. https://doi.org/10.1124/dmd.111.044289.
• Kovacsics D, Patik I, Özvegy-Laczka C. The role of organic anion transporting polypeptides in drug absorption, distribution, excretion and drug-drug interactions. Expert Opin Drug Metab Toxicol. 2017;13(4):409–24. https://doi.org/10.1080/17425255.2017.1253679 This article is a current review discussing the OATP family of transporters and the importance of OATPs in the absorption and distribution of drugs, as well as their role in drug-drug interactions.
Maeda K. Organic anion transporting polypeptide (OATP)1B1 and OATP1B3 as important regulators of the pharmacokinetics of substrate drugs. Biol Pharm Bull. 2015;38(2):155–68. https://doi.org/10.1248/bpb.b14-00767.
• Kulkarni P, Korzekwa K, Nagar S. Intracellular unbound atorvastatin concentrations in the presence of metabolism and transport. J Pharmacol Exp Ther. 2016;359(1):26–36. https://doi.org/10.1124/jpet.116.235689 This article used a 5-compartmental model for the prediction of intracellular concentrations of atorvastation, to understand the influence of transporters on the intracellular concentration.
•• Di L, Breen C, Chambers R, Eckley ST, Fricke R, Ghosh A, et al. Industry perspective on contemporary protein-binding methodologies: considerations for regulatory drug-drug interaction and related guidelines on highly bound drugs. J Pharm Sci. 2017;106(12):3442–52. https://doi.org/10.1016/j.xphs.2017.09.005 This article offers an industry perspective on the current methods used to determine the plasma protein binding of a drug, as well as factors which should be considered in current methodology.
Kochansky CJ, McMasters DR, Lu P, Koeplinger KA, Kerr HH, Shou M, et al. Impact of pH on plasma protein binding in equilibrium dialysis. Mol Pharm. 2008;5(3):438–48. https://doi.org/10.1021/mp800004s.
•• Chan R, De Bruyn T, Wright M, Broccatelli F. Comparing mechanistic and preclinical predictions of volume of distribution on a large set of drugs. Pharm Res. 2018;35(4):11. https://doi.org/10.1007/s11095-018-2360-2 This article compared the use of composition-based tissue: plasma partition coefficient prediction models, as well as preclinical extrapolation for the prediction of the Vss for a set of 152 drugs.
Zou P, Zheng N, Yang YS, Yu LX, Sun DX. Prediction of volume of distribution at steady state in humans: comparison of different approaches. Expert Opin Drug Metab Toxicol. 2012;8(7):855–72. https://doi.org/10.1517/17425255.2012.682569.
Sui XF, Sun J, Li HY, Wang YJ, Liu JF, Liu XH, et al. Prediction of volume of distribution values in human using immobilized artificial membrane partitioning coefficients, the fraction of compound ionized and plasma protein binding data. Eur J Med Chem. 2009;44(11):4455–60. https://doi.org/10.1016/j.ejmech.2009.06.004.
De Buck SS, Sinha VK, Fenu LA, Gilissen RA, Mackie CE, Nijsen MJ. The prediction of drug metabolism, tissue distribution, and bioavailability of 50 structurally diverse compounds in rat using mechanism-based absorption, distribution, and metabolism prediction tools. Drug Metab Dispos. 2007;35(4):649–59. https://doi.org/10.1124/dmd.106.014027.
Funding
The authors acknowledge funding from the National Institutes of Health grants (R01GM104178 and R01GM114369).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Drug Disposition
Rights and permissions
About this article
Cite this article
Holt, K., Nagar, S. & Korzekwa, K. Methods to Predict Volume of Distribution. Curr Pharmacol Rep 5, 391–399 (2019). https://doi.org/10.1007/s40495-019-00186-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-019-00186-5