Abstract
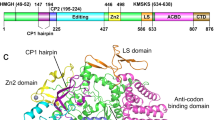
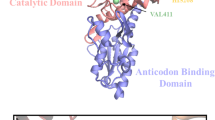
Escherichia coli leucyl-tRNA synthetase (LeuRS) has a large connecting polypeptide (CP1) inserted into its active site. It was demonstrated that the peptide bond between E292–A293 was crucial for the aminoacylation activity of E. coli LeuRS. To investigate the effect of E292 on the function of Escherichia coli LeuRS, E292 was mutated to K, F, S, D, Q and A. These mutations at 292 did not change the specific activity of the amino acid activation reaction. Though the conformational change of these mutants was not detected in CD, their aminoacylation activities were impaired to varying extents. The mutation of E to K decreased the aminoacylation activity to the largest extent. Analysis of the Km values of these mutants for the three substrates showed that the E292 was not involved in the binding of leucine and that all mutants had stronger binding with ATP.
Similar content being viewed by others
References
Amann, E., Ochs, B., and Abel, K. J. (1988). Gene 69: 301–315.
Bradford, M. M. (1976). Anal. Biochem. 72: 248–254.
Burbaum, J. J., and Schimmel, P. (1991). J. Biol. Chem. 266: 16965–16968.
Carter, C. W., Jr. (1993). Annu. Rev. Biochem. 62: 715–748.
Chen, J. F., Guo, N. N., Li, T., Wang, E. D., and Wang, Y. L. (2000). Biochemistry 39: 6726–6731.
Chen, J. F., Li, T., Wang, E. D., and Wang, Y. L. (2001). Biochemistry 40: 1144–1149.
Cusack, S., Yaremchuck, A., and Tukalo, M. (2000). EMBO J. 19: 2351–2361.
Deng, L., Li, B., Shi, J. P., and Wang, Y. L. (1994). Acta Biochim. Biophys. Sinica 26: 223–228.
Eriani, G., Delarue, M., Poch, O., Gangloff, J., and Moras, D. (1990). Nature 26: 223–228.
Fersht, A. R. (1985) In: Enzyme Structure and Mechanism. 2nd ed. New York: W. H. Freeman and Company, pp. 354–358.
Härtlein, M., and Madern, D. (1987). Nucleic Acids Res. 4: 10199–10210.
Ibba, M., and Söll, D. (2000). Annu. Rev. Biochem. 69: 617–650.
Li, T., Wang, E. D., and Wang, Y. L. (1997). Acta Biochim. Biophys. Sinica 29: 591–596.
Li, Y., Wang, E. D., and Wang, Y. L. (1998). Sci. China 41: 225–231.
Li, T., Guo, N. N., Xia, X., Wang, E. D., and Wang, Y. L. (1999a). Biochemistry 38: 13063–13069.
Li, T., Li, Y., Guo, N. N., Wang, E. D., and Wang, Y. L. (1999b). Biochemistry 38: 9084–9088.
Shiba, K., and Schimmel, P. (1992). Proc. Natl. Acad. Sci. USA 89: 1880–1884.
Starzyk, R. M., Webster, T. A., and Schimmel, P. (1987). Science 237: 1614–1618. —-
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, X., Wang, ED. E292 Is Important for the Aminoacylation Activity of Escherichia coli Leucyl-tRNA Synthetase. J Protein Chem 22, 71–76 (2003). https://doi.org/10.1023/A:1023071928587
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1023071928587