Abstract
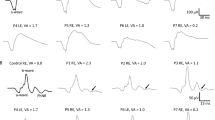
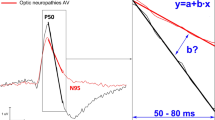
Purpose: To assess the retinal function in patients with von Hippel-Lindau disease (VHL). Patients: Studies were undertaken in 12 patients (17 eyes) with detected VHL gene mutation and 12 normal healthy controls (17 eyes). Methods: Pattern ERG (PERG), standard flash electroretinogram (ERG) recordings were performed in accordance with the International Society for Clinical Electrophysiology of Vision (ISCEV) standards. Results: In VHL patients, electrophysiological statistically significant changes were found. In PERG examination, increased latency of P50 was found in the total VHL group (p<0.02) and in the VHL subgroup with retinal angiomas (p<0.04). In ERG examination, photopic b-wave latency was increased in the total VHL group (p<0.03) and also in the VHL subgroup without retinal angiomas (p<0.05). In OPs, latency increase of OP2, OP3 waves and reduced amplitude of OP3 wave in the total VHL group (OP2 latency, p<0.05; OP3 latency, p<0.01; OP3 amplitude, p<0.03) and in the VHL subgroup with retinal angiomas (OP2 latency, p<0.02; OP3 latency, p<0.008; OP3 amplitude, p<0.02) were obtained. Conclusions: It can be hypothesized that dysfunction of the inner retinal layer is present in individuals with VHL disease even in patients without retinal angiomas.
Similar content being viewed by others
References
Maher ER, Iselius L, Yates JRW, et al. Von Hippel-Lindau disease: a genetic study. J Med Genet 1991; 28: 443–7.
Fearon E. Human cancer syndromes: clues to the origin and nature of cancer. Science 1997; 278: 1043–50.
Latif F, Tory K, Gnarra J, et al. Identyfication of the von Hippel-Lindau disease tumor suppressor gene. Science 1993; 260: 1317–20.
Chan Chi-Chao, Vortmeyer AO, Chew EY, Green WR, Matteson DM, Shen De F, Linehan WM, Lubensky IA, Zhuang Z. VHL gene deletion and enhanced VEGF gene expression detected in the stromal cells of retinal angioma. Arch Ophthalmol 1999; 117: 625–30.
Siemeister G, Weindel K, Mohrs K, et al. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res 1996; 56: 2299–301.
Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA 1996; 93: 10595–99.
Grossniklaus H, Thomas J, Vigneswaran N, Jarret W III. Retinal hemangioblastoma: a histologic, immunohistochemical, and ultrastructural evaluation. Ophthalmology 1992; 99: 140–5.
Nicholson D. Capillary haemangioma of the retina and von Hippel-Lindau disease. In: Ogden T, Schachat A, eds. Retina Vol 1. St Louis, MO: Mosby; 1994: 633–40.
Webster A, Maher E, Moore A. Clinical characteristics of ocular angiomatosis in von Hippel-Lindau disease and correlation with germline mutation. Arch Ophthalmol 1999: 117: 371–8.
Green WR. Retina: capillary hemangioma. In Spencer WH, ed. Ophthalmic pathology: An Atlas and Textbook. Philadelphia, PA: WB Saunders Co, 1996: 709–18.
Jakobiec FA, Font RL, Johnson FB. Angiomatosis retinae: An ultrastructural study and lipid analysis. Cancer 1976; 38: 2042–56.
Marmor MF, Zrenner E. Standard for clinical electroretinography (1999 update). Doc Ophthalmol 1999; 97: 143–56.
Bach M, Hawlina M, Holder GE, Marmor MF, Meigen T, Vaegan, Miyake Y. Standard for pattern electroretinography. Doc Ophthalmol 2000; 101: 35–49.
Maffei L, Fiorentini A. Electroretinographic responses to alternative gratings before and after section of the optic nerve. Science 1981; 211: 953–5.
Arden GB, Vaegan, Hoog CR. Clinical and experimental evidence that pattern electroretinogram (PERG) is generated in more proximal layers than the focal electroretinogram (FERG). Ann NY Acad Sci 1982; 388: 580–607.
Holder EG. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retinal Eye Res 2001; 20(4): 531–61.
Xu XJ, Karwoski J. Current source density analysis of retinal field potentials. II Pharmacological analysis of the b-wave and M-wave. J Neurophysiol 1994; 72: 96–105.
Dick E, Miller RF. Light-evoked potassium activity in the mudpuppy retina. Its relationship to the b-wave of the electroretinogram. Brain Res 1978;154: 388–94.
Joussen MA, Kirchhof B. Solitary peripapillary hemangioblastoma. A histopathological case report. Acta Ophthalmol Scand 2001; 79: 83–7.
Ogden TE. The oscillatory waves of the primate electroretinogram. Vision Res 1973; 13: 1059–74.
Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retinal Eye Res 1998; 17: 485–521.
Parisi V, Uccioli L, Monticone G, Parisi L, Manni G, et al. Electrophysiological assessment of visual function in IDDM patients. Electroencephalogr Clin Neurophysiol 1997; 104: 171–9.
Chung NH, Kim SH, Kwak MS. The electroretinogram sensitivity in patients with diabetes. Korean J Ophthalmol 1993; 7(2): 43–7.
Yoshida A, Kojima M, Ogasawara H, Ishiko S. Oscillatory potentials and permeability of the blood-retinal barrier in noninsulin-dependent diabetics patients without retinopathy. Ophthalmology 1991; 98(8): 1266–71.
Van der Torren K, Mulder P. Comparison of the second and third oscillatory potentials with oscillatory potential power in early diabetic retinopathy. Doc Ophthalmol 1993; 83(2): 111–8._
Peppe A, Stanzione P, Pierelli F, De Andelis D, Pierantozzi M, Bernardi G. Visual alterations in de novo Parkinson's disease: Pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology 1995; 45: 1144–8.
Bartel P, Blom M, Robinson M, van der Meyden C, Sommer DK, Becker P. The effect of levodopa and haloperidol on flash and pattern ERGs and VEPs in normal humans. Doc Ophthalmol 1990; 76(1): 55–64.
Graham SL, Klistorner A. Electrophysiology: A review of signal origins and applications to investigating glaucoma. Austr New Zealand J Ophthalmol 1998; 26: 71–85.
Reuther K, Kelner U. Inner retinal function in hereditary retinal dystrophies. Acta Anat 1998; 162: 169–77.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lubiński, W., Krzystolik, K., Cybulski, C. et al. Retinal function in the von Hippel-Lindau disease. Doc Ophthalmol 106, 271–280 (2003). https://doi.org/10.1023/A:1022919631288
Issue Date:
DOI: https://doi.org/10.1023/A:1022919631288