Abstract
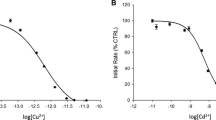
As a possible probe for metal activation of calcineurin, Tb3+ was tested for effects on calcineurin activity. Calcineurin was activated by Tb3+ with the following kinetic parameters estimated: k cat = 0.78 ± 0.02 sec−1, K m(pNPP) = 32.6 ± 1.8 mM, and K act(Tb3+) = 0.08 ± 0.03 mM. Terbium luminescence was demonstrated in the presence of the heterodimer of calcineurin and exploited to localize the binding of exogenous metal to the enzyme active site. Exogenous Mn2+ reduced luminescence, although the affinity of calcineurin for Tb3+ seemed to be greater. Putative active-site ligands, such as para-nitrophenol and a synthetic peptide from the autoinhibitory region, reduced the luminescence of terbium. Collectively, these data suggested that Tb3+ was binding directly at the active site of calcineurin, with the corollary that exogenous activating metal (Mn2+) binds at the active site of the enzyme. These data support the hypothesis that activating, exogenous divalent metal participates directly in catalysis.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, B.L., Jurado, L.A. Activation of Calcineurin by the Trivalent Metal Terbium. J Protein Chem 17, 473–478 (1998). https://doi.org/10.1023/A:1022574719239
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1022574719239