Abstract
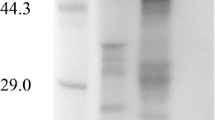
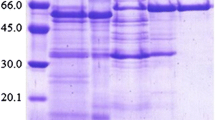
Two intracellular β-glucosidases (E.C. 3.2.1.21) were purified from the filamentous fungus Neurospora crassa, mutant cell-1 (FGSC no. 4335) and characterized. The extent of purification were 2.55- and 28.89-fold for β-glucosidase A and β-glucosidase B, respectively. β-Glucosidase A was a dimeric protein, and B a monomeric protein, with molecular masses of 178 and 106 kDa, respectively. Both isoenzymes were glycoproteins with relatively high carbohydrate contents (β-glucosidase A, 29.2%; β-glucosidase B, 34.2%). The isoelectric points determined by IEF were 6.27 and 4.72, respectively. pH optima for activity were determined to be 5.0 and 5.5, and temperature optima to be 55 and 60 °C, for β-glucosidases A and B, respectively. Both purified β-glucosidases. especially β-glucosidase B, showed relatively high stability against pH and temperature. Both enzymes were stable in the pH range of 5.0–9.0. The activities were completely retained up to 48 h at temperatures below 40 °C. At higher temperatures, enzymes were relatively unstable and lost their activities at 60 °C after 24 h. Both β-glucosidases were highly activated by CuCl2, and inhibited by SnCl2 and KMnO4. Hg2+ and Ag+ also inhibited severely β-glucosidase B. The K m and V max values of the isoenzymes against cellobiose as substrate were 1.50 mM and 12.2μmol min−1 mg−1 for β-glucosidase A and 2.76 mM and 143.5 μmol min−1 mg−1 for β-glucosidase B.
Similar content being viewed by others
References
Atlas, R.M. 1997 In Handbook of Microbiological media, 2nd edn. ed. Parks L.C. b. 1529. USA: CRC Press Inc. ISBN 0-84932638-9.
Bhat, M.K. & Bhat, S. 1997 Cellulose degrading enzymes and their potential industrial applications. Biotechnology Advances 15, 583-620.
Cao, W. & Crawford, D.L. 1993 Purification and some properties of β-glucosidase from the ectomycorrhizal fungus Pisolithus tinctorius strain SMF. Canadian Journal of Microbiology 39, 125-129.
Coughlan, M.P. 1985 The properties of fungal and bacterial cellulases with comments on their production and application. Biotechnology and Genetic Engineering Reviews 3, 39-109.
Dekker, R.F.H. 1981 Induction, localization and characterization of β-glucosidases produced by Monila. Journal of General Microbiology 127, 177-184.
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. & Smith, F. 1956 Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28, 350-356.
Eberhart, B.M., Beck, R.S. & Goolsby, K.M. 1977 Cellulase of Neurospora crassa. Journal of Bacteriology 130, 181-186.
Fincham, J.R.S. 1989 Transformation in fungi. Microbiological Reviews 53, 148-170.
Gueguen, Y., Chemardin, P., Arnaud, A. & Galzy, P. 1995 Purification and characterization of an intracellular β-glucosidase from Botrytis cinerea. Enzyme and Microbial Technology 17, 900-906.
Hames, B.D. 1990 In Gel Electrophoresis of Proteins-A Practical Approach eds. Hames, B.D. & Rickwood, D. pp. 1-86. IRL Press at Oxford University Press. ISBN 0-19963075-5.
Kadam, S.K. & Demain, A.L. 1989 Addition of cloned β-glucosidase enhances the degradation of crystalline cellulose by Clostridium thermocellum cellulase complex. Biochemical and Biophysical Research Communications 161, 706-711.
Kunst, A., Draeger, B. & Ziegenhorn, J. 1984 Colorimetric methods with glucose oxidase and peroxidase. In Methods of Enzymatic Analysis. Bergmeyer, H.U., Bergmeyer, J.U. & Grassl, M. eds. vol VI. pp. 178-185. Deerfield Beach, FL: Verlag Chemie. ISBN 3-52726046-3.
Le Traon-Masson, M.P. & Pellerin, P. 1998 Purification and characterization of two ?-D-glucosidases from an Aspergillus niger enzyme preparation: affinity and specificity toward glucosylated compounds characteristic of the processing of fruits. Enzyme Microbial Technology 22, 374-382.
Li, X. & Calzo, RE. 1991 Purification and characterization of an extracellular β-glucosidase from the rumen fungus Neocallimastix frontalis EB 188. Enzyme Microbial Technology 13, 622-628.
Mega, T. & Matsushima, Y. 1997 Comparative studies of three exo-?-glycosidases of Aspergillus oryzae. Journal of Biochemistry (Tokyo) 85, 335-341.
Mishra, N.C. 1991 Genetics and molecular biology of Neurospora crassa-a review. Advances in Genetics 29, 2-62.
Perkins, D.D., Radford, A., Newmeyer, D. & Bjorkman, M. 1982 Chromosomal loci of Neurospora crassa. Microbiological Reviews, 46, 426-570.
Rashid, M.H. & Siddiqui, K.S. 1996 The stability of extracellular β-glucosidase from Aspergillus niger is significantly enhanced by noncovalently attached polysaccharides. Folia Microbiologica 41, 341-346.
Sasaki, I. & Nagayama, H. 1995 Purification and characterization of β-glucosidase from Botrytis cinerea. Bioscience, Biotechnology and Biochemistry 59, 100-101.
Siddiqui, K.S., Rashid, M.H., Shemsi, A.M. & Rajoka, M.I. 1994 A simple and nondestructive method for the separation of polysaccharides from β-glucosidase produced extracellularely by Aspergillus niger. Enzyme and Microbial Technology 16, 912-917.
Singh, A. & Hayashi, K. 1995 Microbial cellulases: Protein architecture, molecular properties, and biosynthesis. Advances in Applied Microbiology 40, 1-35.
Watanabe, T., Sato, T., Yoshioka, S., Koshijima, T. & Kuwahara, M. 1992 Purification and properties of Aspergillus niger β-glucosidase. European Journal of Biochemistry 209, 651-659.
Yazdi, M.T., Radford, A., Keen, J.N. & Woodward, J.R. 1990a Cellulase production by Neurospora crassa: Purification and characterization of cellulotic enzymes. Enzyme and Microbial Technology 12, 120-123.
Yazdi, M.T., Woodward J.R. & Radford, A. 1990b The cellulase complex of Neurospora crassa: activity, stability and release. Journal of General Microbiology 136, 1313-1319.
Yazdi, M.T., Woodward, J.R. & Radford, A. 1990c Cellulase production by Neurospora crassa: The enzymes of the complex and their regulation. Enzyme and Microbial Technology 12, 116-119.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yazdi, M.T., Khosravi, A.A., Nemati, M. et al. Purification and characterization of two intracellular β-glucosidases from the Neurospora crassa mutant cell-1 . World Journal of Microbiology and Biotechnology 19, 79–84 (2003). https://doi.org/10.1023/A:1022531706036
Issue Date:
DOI: https://doi.org/10.1023/A:1022531706036