Abstract
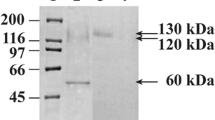
A new α-glucosidase from Shiraia sp. SUPER-H168 under solid-state fermentation was purified by alcohol precipitation and anion-exchange and by gel filtration chromatography. The optimum pH and temperature of the purified α-glucosidase were 4.5 and 60 °C, respectively, using p-nitrophenyl-α-glucopyranoside (α-pNPG) as a substrate. Ten millimoles of sodium dodecyl sulfate, Fe2+, Cu2+, and Ag+ reduced the enzyme activity to 0.7, 7.6, 26.0, and 6.2 %, respectively, of that of the untreated enzyme. The K m, V max, and k cat/K m of the α-glucosidase were 0.52 mM, 3.76 U mg−1, and 1.3 × 104 L s−1 mol−1, respectively. K m with maltose was 0.62 mM. Transglycosylation activities were observed with maltose and sucrose as substrates, while there was no transglycosylation with trehalose. DNA and its corresponding full-length cDNA were cloned and analyzed. The α-glucosidase coding region consisted of a 2997-bp open reading frame encoding a 998-amino acid protein with a 22-amino acid signal peptide; one 48-bp intron was located. The α-glucosidase was a monomeric protein with a predicted molecular mass of 108.2 kDa and a predicted isoelectric point of 5.08. A neighbor-joining phylogenetic tree demonstrated that Shiraia sp. SUPER-H168 α-glucosidase is an ascomycetes α-glucosidase. This is the first report of α-glucosidase from a filamentous fungus that had good glycoside hydrolysis with maltose and α-pNPG, transglycosylation and conversion activity of maltose into trehalose.







Similar content being viewed by others
References
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350. doi:10.1093/bioinformatics/btq662
Bothast RJ, Schlicher MA (2005) Biotechnological processes for conversion of corn into ethanol. Appl Microbiol Biotechnol 67:19–25. doi:10.1007/s00253-004-1819-8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1006/abio.1976.9999
Cai YJ, Liang XH, Liao XR, Ding YR, Sun J, Li XH (2010a) High-yield hypocrellin a production in solid-state fermentation by shiraia sp SUPER-H168. Appl Biochem Biotechnol 160:2275–2286. doi:10.1007/s12010-009-8728-3
Cai YJ, Wei ZY, Liao XR, Ding YR, Sun J (2010b) Characterization of three extracellular polysaccharides from shiraia sp super-H168 under submerged fermentation. Carbohydr Polym 82:34–38. doi:10.1016/j.carbpol.2010.04.016
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The carbohydrate-active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238. doi:10.1093/nar/gkn663
Chiba S (1997) Molecular mechanism in alpha-glucosidase and glucoamylase. Biosci Biotechnol Biochem 61:1233–1239
da Silva TM et al (2009) Purification and biochemical characterization of a novel alpha-glucosidase from aspergillus niveus. Anton Leeuw Int J G 96:569–578. doi:10.1007/s10482-009-9372-1
Ezeji TC, Bahl H (2006) Purification, characterization, and synergistic action of phytate-resistant alpha-amylase and alpha-glucosidase from geobacillus thermodenitrificans HRO10. J Biotechnol 125:27–38. doi:10.1016/j.jbiotec.2006.02.006
Frandsen TP, Svensson B (1998) Plant alpha-glucosidases of the glycoside hydrolase family 31. Molecular properties, substrate specificity, reaction mechanism, and comparison with family members of different origin. Plant Mol Biol 37:1–13. doi:10.1023/a:1005925819741
Frandsen TP, Lok F, Mirgorodskaya E, Roepstorff P, Svensson B (2000) Purification, enzymatic characterization, and nucleotide sequence of a high-isoelectric-point alpha-glucosidase from barley malt. Plant Physiol 123:275–286. doi:10.1104/Pp.123.1.275
Frohman MA, Dush MK, Martin GR (1988) Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A 85:8998–9002. doi:10.1073/pnas.85.23.8998
Giannesi GC, Polizeli M, Terenzi HF, Jorge JA (2006) A novel alpha-glucosidase from chaetomium thermophilum var. coprophilum that converts maltose into trehalose: purification and partial characterisation of the enzyme. Process Biochem 41:1729–1735. doi:10.1016/j.procbio.2006.03.017
Guex N, Peitsch MC (1997) SWISS-MODEL and the swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723. doi:10.1002/elps.1150181505
Henrissat B (1991) A classification of glycosyl hydrolases based on amino-acid-sequence similarities. Biochem J 280:309–316
Henrissat B, Bairoch A (1993) New families in the classification of glycosyl hydrolases based on amino-acid-sequence similarities. Biochem J 293:781–788
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644. doi:10.1016/s0959-440x(97)80072-3
Kato N, Suyama S, Shirokane M, Kato M, Kobayashi T, Tsukagoshi N (2002) Novel alpha-glucosidase from aspergillus nidulans with strong transglycosylation activity. Appl Environ Microbiol 68:1250–1256. doi:10.1128/aem.68.3.1250-1256.2002
Kita A, Matsui H, Somoto A, Kimura A, Takata M, Chiba S (1991) Substrate-specificity and subsite affinities of crystalline alpha-glucosidase from aspergillus-niger. Agric Biol Chem 55:2327–2335
Krohn BM, Lindsay JA (1991) Purification and characterization of a thermostable alpha-glucosidase from a bacillus-subtilis high-temperature growth transformant. Curr Microbiol 22:273–278. doi:10.1007/bf02091954
Kurihara H, Ando J, Hatano M, Kawabata J (1995) Sulfoquinovosyldiacylglycerol as an alpha-glucosidase inhibitor. Bioorg Med Chem Lett 5:1241–1244. doi:10.1016/0960-894x(95)00196-z
Lacerda LG, da Silva Carvalho Filho MA, Demiate IM, Bannach G, Ionashiro M, Schnitzler E (2008) Thermal behaviour of corn starch granules under action of fungal alpha-amylase. J Therm Anal Calorim 93:445–449. doi:10.1007/s10973-006-8273-z
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Legin E, Copinet A, Duchiron F (1998) Production of thermostable amylolytic enzymes by thermococcus hydrothermalis. Biotechnol Lett 20:363–367. doi:10.1023/a:1005375213196
Liang XH, Cai YJ, Liao XR, Wu K, Wang L, Zhang DB, Meng Q (2009) Isolation and identification of a new hypocrellin a-producing strain shiraia sp SUPER-H168. Microbiol Res 164:9–17. doi:10.1016/j.micres.2008.08.004
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–663
Marin D, Linde D, Lobato MF (2006) Purification and biochemical characterization of an alpha-glucosidase from xanthophyllomyces dendrorhous. Yeast 23:117–125. doi:10.1002/yea.1345
Maruta K, Nakada T, Kubota M, Chaen H, Sugimoto T, Kurimoto M, Tsujisaka Y (1995) Formation of trehalose from maltooligosaccharides by a novel enzymatic system. Biosci Biotechnol Biochem 59:1829–1834
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Nishimoto T et al (1995) Existence of a novel enzyme converting maltose into trehalose. Biosci Biotechnol Biochem 59:2189–2190
Ramani G, Meera B, Vanitha C, Rajendhran J, Gunasekaran P (2015) Molecular cloning and expression of thermostable glucose-tolerant beta-glucosidase of penicillium funiculosum NCL1 in pichia pastoris and its characterization. J Ind Microbiol Biotechnol 42:553–565. doi:10.1007/s10295-014-1549-6
Shi YJ, Xu XQ, Zhu Y (2009) Optimization of verticillium lecanii spore production in solid-state fermentation on sugarcane bagasse. Appl Microbiol Biotechnol 82:921–927. doi:10.1007/s00253-009-1874-2
Tagami T, Yamashita K, Okuyama M, Mori H, Yao M, Kimura A (2013) Molecular basis for the recognition of long-chain substrates by plant alpha-glucosidases. J Biol Chem 288:19296–19303. doi:10.1074/jbc.M113.465211
Takewaki S, Kimura A, Kubota M, Chiba S (1993) Substrate-specificity and subsite affinities of honeybee alpha-glucosidase.2. Biosci Biotechnol Biochem 57:1508–1513
Wongchawalit J et al (2006) Purification and characterization of alpha-glucosidase I from Japanese honeybee (apis cerana japonica) and molecular cloning of its cDNA. Biosci Biotechnol Biochem 70:2889–2898. doi:10.1271/bbb.60302
Yamasaki Y, Fujimoto M, Kariya J, Konno H (2005) Purification and characterization of an alpha-glucosidase from germinating millet seeds. Phytochemistry 66:851–857. doi:10.1016/j.phytochem.2005.02.024
Yang YC, Ding YR, Liao XR, Cai YJ (2013) Purification and characterization of a new laccase from shiraia sp.SUPER-H168. Process Biochem 48:351–357. doi:10.1016/j.procbio.2012.12.011
Yang Y, Guan Z, Ding Y, Liao X, Cai Y (2014) Fermentation optimization, cloning and sequence analysis of the laccase gene from shiraia sp. SUPER-H168. Ann Microbiol. doi:10.1007/s13213-014-0893-0
Zhang Y-k, Li W, Wu K-y, Chen G-g, Liang Z-q (2011) Purification and characterization of an intracellular -glucosidase with high transglycosylation activity from a. Niger M-1. Prep Biochem Biotechnol 41:201–217. doi:10.1080/10826068.2011.547384
Zhou C, Xue YF, Ma YH (2015) Evaluation and directed evolution for thermostability improvement of a GH 13 thermostable alpha-glucosidase from thermus thermophilus TC11. BMC Biotechnol 15:97. doi:10.1186/s12896-015-0197-x
Acknowledgements
This work was financially supported by the National High Technology and special funds for science and technology innovation from the Science and Technology Department of Jiangsu province (Grant No. BY2010117), the National Natural Science Foundation of China (Grant No. 21275066), and fundamental research of doctor of philosophy at 2014 (Grant No. 2050205), Project of graduate student scientific research and innovation of Jiangsu province at 2015 (Grant No. 205020502), Six talent peak program at 2016 (Grant No. SWYY-126).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, R., Deng, H., Guan, Z. et al. Purification, characterization and gene analysis of a new α-glucosidase from shiraia sp. SUPER-H168. Ann Microbiol 67, 65–77 (2017). https://doi.org/10.1007/s13213-016-1238-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-016-1238-y