Abstract
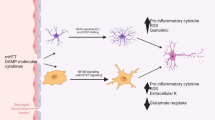
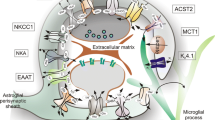
Astrocytes are ubiquitous in the brain and have multiple functions. It is becoming increasingly clear that they play an important role in monitoring the neuromicroenvironment in CNS and in information processing or signaling in the nervous system in normal conditions and respond to CNS injuries in a gradual and varied way. It is still debated whether such reactions are beneficial or detrimental. It was believed that reactive astrogliosis observed in most neurological disorders may regulate the removal of toxic compounds produced by damaged neurons and support neuronal growth by releasing trophic factors. However it was also suggested that astrocytes contribute to a decline of neurologic function, for example by accumulation and release of excitotoxic aminoacids after ischemia and oxidative stress, formation of epileptogenic scars in response to CNS injury and metabolism of protoxins to potent toxins. In a number of metabolic diseases astrocytes, not neurons, may be the primary target. The astrocyte's role in normal and pathological conditions will be discussed in the light of recent information about their metabolism, receptor distribution and release.
Similar content being viewed by others
REFERENCES
Mucke, L., and Eddleston, M. 1993. Astrocytes in infectious and immunemediated diseases of the central nervous system. FASEB J. 7:1226–1232.
Giulian, D. 1993. Reactive glia as rivals in regulating neuronal survival. Glia 7:102–110.
Ferrara, N., Ousley, F., and Gospodarowicz, D. 1988. Bovine brain astrocytes express basic fibroblast growth factor, a neurotropic and angiogenic mitogen. Brain. Res. 462:223–232.
Varon, S., Manthorpe, M., and Adler, R. 1979. Cholinergic neuronotrophic factors: I. Survival, neurite outgrowth and choline acetyltransferase activity in monolayer cultures from chick embryo ciliary ganglia. Brain. Res. 173:29–45.
Visentin, M., Salmona, M., and Tacconi, M. T. 1995. Reye's and Reye-like syndromes drug-related diseases? (causative agents, etiology, pathogenesis and therapeutic approaches). Drug Metab. Rev. 27:517–539.
Muller, C. M. 1992. A role for glial cells in activity-dependent central nervous system plasticity? Review and hypothesis. Int. Rev. Neurobiol. 34:215–281.
Barres, B. A., Chun, L. L. Y., and Corey, D. P. 1990. Ion channels in vertebrate glia. Annu. Rev. Neurosci. 13:441–474.
Glowinski, J., Marin, P., Tence, M., Stella, N., Giaume, C., and Premont, J. 1994. Glial receptors and their intervention in astrocyto-astrocytic and astrocyto-neuronal interactions. Glia 8:202–208.
Chiu, S. Y., and Kriegler, S. 1994. Neurotransmitter-mediated signaling between axons and glial cells. Glia 11:191–200.
Norenberg, M. D. 1994. Astrocyte responses to CNS injury. J. Neuropathol. Exp. Neurol. 53:213–220.
Landis, D. M. D. 1994. The early reactions of non-neuronal cells to brain injury. Annu. Rev. Neurosci. 17:133–151.
Schipper, H. M. 1996. Astrocytes, brain aging, and neurodegeneration. Neurobiol. Aging 17:467–480.
Eddleston, M., and Mucke, L 1993. Molecular profile of reactive astrocytes-implications for their role in neurologic disease. Neuroscience 54:15–36.
Vernadakis, A. 1988. Neuron-glia Interrelations. Int. Rev. Neurobiol. 30:149–224.
Wilkins, G. P., Marriott, D. R., and Cholewiski, A. J. 1990. Astrocyte heterogeneity. Trends Neurosci. 13:43–46.
Giaume, C., Fromaget, C., el-Aoumari, A., Cordier, J., Glowinski, J., and Gros, D. 1991. Gap junctions in cultured astrocytes: single channel current and characterization of channel-forming protein. Neuron 6:133–143.
Giaume, C., Marin, P., Cordier, J., and Premont, J. 1991. Adrenergic regulation of intercellular communications between cultured striatal astrocytes from the mouse. Proc. Natl. Acad. Sci. USA 88:5577–5581.
DeVries, S. H., and Schwartz, E. A. 1989. Modulation of an electrical synapse between solitary path of catfish horizontal cells by dopamine and second messengers. J. Physiol, Lond. 414:351–375.
Muller, T., and Kettenmann, H. 1995. Physiology of Bergmann glial cells. Int. Rev. Neurobiol. 38:341–359.
Rosenman, S. J., Shrikant, P., Dubb, L., Benveniste, E. N., and Ransohoff, R. M. 1995. Cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and astrocytoma cell lines. J. Immunol. 154:1888–1899.
Spector, R. 1988. Fatty acid transport through the blood-brain barrier. J. Neurochem. 50:639–643.
Magret, V., Elkhalil, L., Nazih-Sanderson, F., Martin, F., Bourre, J. M., Fruchart, J. C., and Delbart, C. 1996. Entry of polyunsaturated fatty acids into the brain: evidence that high-density lipoprotein-induced methylation of phosphatidylethanolamine and phospholipase A2 are involved. Biochem. J. 316:805–811.
Moore, S. A., Yoder, E., Murphy, S., Dutton, G. R., and Spector, A. A. 1991. Astrocytes, not neurons, produce docosahexaenoic acid (22:6w-3) and arachidonic acid (20:4w-6). J. Neurochem. 56:518–524.
Varon, S. S., and Somjen, G. G. 1979. Neuron-glia interactions. Neurosci. Res. Progr. Bull. 17:1–239.
Walz, W., and Hertz, L. 1983. Functional interactions between neurons and astrocytes. II. Potassium homeostasis at the cellular level. Progr. Neurobiol. 20:133–183.
Norenberg, M. D., Hertz, L., and Shousboe, A. (eds) 1988: The biochemical pathology of astrocytes, NY, Alan R. Liss.
Lee, S. C., Liu, W., Dickson, D. W., Brosnan, C. F., and Berman, J. W. 1993. Cytokine production by human fetal microglia and astrocytes. J. Immunol. 150:2659–2667.
Yu, N., Maciejewski-Lenoir, D., Bloom, F. E., and Magistretti, P. J. 1995. Tumor necrosis factor alfa and interleukin-1 alfa enhance glucose utilization by astrocytes: involvement of phospholipase A2. Mol. Pharmacol. 48:550–558.
Simmons, M. L., and Murphy, S. 1992. Induction of nitric oxide synthase in glial cells. J. Neurochem. 59:897–905.
Kugler, P., and Drenckhahn, D. 1996. Astrocytes and Bergmann glia as an important site of nitric oxide synthase I. Glia 16:165–173.
Hu, S., Sheng, W. S., Peterson, P. K., and Chao, C. C. 1995. Differential regulation by cytokines of human astrocyte nitric oxide production. Glia 15:491–494.
Yu, N., Martin, J-L., Stella, N., and Magistretti, P. J. 1993. Arachidonic acid stimulates glucose uptake in cerebral cortical astrocytes. Proc. Natl. Acad. Sci. USA 90:4042–4046.
Sorg, O., and Magistretti, P. J. 1991. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain. Res. 563:227–233.
Sorg, O., and Magistretti, P. J. 1992. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J. Neurosci. 12:4923–4931.
Pellerin, L., and Magistretti, P. J. 1994. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 91:10625–10629.
Sorg, O., Pellerin, L., Stolz, M., Beggah, S., and Magistretti, P. J. 1995. Adenosine triphosphate and arachidonic acid stimulate glycogenolysis in primary cultures of mouse cerebral cortex astrocytes. Neurosci. Lett. 188:109–112.
Dringen, R., Gebhardt, R., and Hamprecht, B. 1993. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain. Res. 623:208–214.
Auestad, N., Korsak, R. A., Morrow, J. W., and Edmond, J. 1991. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J. Neurochem. 56:1376–1386.
Murphy, M. G., Jollimore, C., Crocker, J. F. S., and Her, H. 1992. Beta-oxidation of [1-14C] palmitic acid by mouse astrocytes in primary culture: effects of agents implicated in the encephalopathy of Reye's syndrome. J. Neurosci. Res. 33:445–454.
Warshaw, J. B., and Terry, M. L. 1976. Cellular energy metabolism during fetal development. VI Fatty acid oxidation by developing brain. Dev. Biol. 52:161–166.
Bossi, E., Zuppinger, K., Siegrist, H. P., Wiesmann, U., and Herschkowitz, N. 1982. Age-dependent utilization of D-beta-OH butyrate and oleic acid as glucose substitutes by neonatal mouse brain cell cultures. Pediatr. Res 16:579–582.
Edmond, J., Robbins, R. A., Bergstrom, J. D., Cole, R. A., and DeVellis, J. 1987. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes and oligodendrocytes from developing brain in primary culture. J. Neurosci. Res 18:551–561.
Flynn, C. J., and Wecker, L. 1987. Concomitant increases in the levels of choline free fatty acids in rat brain: evidence supporting the seizure-induced hydrolysis of phosphatidylcholine. J. Neurochem. 48:1178–1184.
Chan, P. H., Longar, S., and Fishman, R. A. 1983. Phospholipid degeneration and edema development in cold-injured rat brain. Brain. Res. 277:329–337.
Stella, N., Tence, M., Glowinski, J., and Premont, J. 1994. Glutamate-evoked release of arachidonic acid from mouse brain astrocytes. J. Neurosci. 14:568–575.
Kimelberg, H. K., Goderie, S. K., Higman, S., Pang, S., and Waniewski, R. A. 1990. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 10:1583–1591.
Mucke, L., Oldstone, M. B. A., Morris, J. C., and Nerenberg, M. I. 1991. Rapid activation of astrocyte specific expression of GFAP-lacZ transgene by focal injury. New Biol. 3:465–474.
Ankarcrona, M., Dypbukt, J. M., Bonfoco, E., Zhivotovsky, B., Orrenius, S., Lipton, S. A., and Nicotera, P. 1995. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 15:961–973.
Barker, J. E., Bolanos, J. P., Land, J. M., B. Clark, J., and Heales, S. J. R. 1996. Glutathione protects astrocytes from peroxynitrite-mediated mitochondrial damage: implications for neuronal/astrocytic trafficking and neurodegeneration. Dev. Neurosci 18:391–396.
Bolanos, J. P., Heales, S. J. R., Land, J. M., and Clark, J. B. 1995. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J. Neurochem. 64:1965–1972.
Makar, T. K., Nedergaard, M., Preuss, A., Gelbard, A. S., Perumal, A. S., and Cooper, A. J. L. 1994. Vitamin E, ascorbate, glutathione, glutathione disulfide and enzymes of glutathione metabolism in culture of chick astrocytes and neurones: evidence that astrocytes play an important antioxidant role in antioxidative processes in the brain. J. Neurochem. 62:45–53.
Fukuda, K., Panter, S. S., Sharp, F. R., and Noble, L. J. 1995. Induction of heme oxygenase-1 (HO-1) after traumatic brain injury in the rat. Neurosci. Lett. 199:127–130.
Smith, G. M., Rutishauser, U., Silver, J., and Miller, M. H. 1990. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev. Biol. 138–2:377–390.
Tiveron, M. C., Barboni, E., Pliego-Rivero, F. B., Gormley, A. M., Seeley, P. J., Grosveld, F., and Morris, R. 1992. Selective inhibition of neurite outgrowth on mature astrocytes by Thy-1 glycoprotein. Nature 355(6362):745–748.
Rowe, P. C., Valle, D., and Brusilow, S. W. 1988. Inborn errors of metabolism in children referred with Reye's syndrome. A changing pattern. J. Am. Med. Assoc. 260:3167–70.
Green, A., and Hall, S. M. 1992. Investigation of metabolic disorders resembling Reye's syndrome. Arch. Dis. Child. 67:1313–1317.
Glasgow, J. F. T., and Moore, R. 1993. Reye's syndrome 30 year on. Br. Med. J. 307:950–951.
Tonsgard, J. H. 1985. Urinary dicarboxylic acids in Reye's syndrome. J. Pediatr. 107:79.
Olson, J. E., Holtzman D., Sankar R., Lawson C., and Rosenberg, R. 1989. Octanoic acid inhibits astrocyte volume control: implications for cerebral edema in Reye's syndrome. J. Neurochem. 52:1197–1202.
Shigenaga, M. K., Hagen, T. M., and Ames, B. N. 1994. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 91:10771–10778.
Sohal, R. S., Ku H. H., Agarwal, S., Forster, M. J., and Lal H. 1994. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Aging. Dev. 74:121–133.
Connor, J. R., Menzles, S., St. Martin, S. M., and Mufson, E. J. 1990. Cellular distribution of transferrin, ferritin and iron in normal and aged human brains. J. Neurosci. 27:595–611.
Jellinger, P., Paulus, W., Grundke-Iqbal, I., Riederer, P., and Youdim, M. B. 1990. Brain iron and ferritin in Parkinson's and Alzheimer's disease. J. Neural. Trasm. Park. Dis. Dement. Sect. 2:327–340.
Saura, J., Luque, J. M., Cesura, A. M., Da Prada, M. D., Chan-Palay, V., Huber, G., Loffler, J., and Richards, J. G. 1994. Increased monoamine oxidate B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 62:15–30.
Wang, J., Lieberman, D., Tabubo, H., Finberg, J. P. M., Oldfield, E. H., and Bankiewicz, K. 1994. Effects of gliosis on dopamine metabolism in rat striatum. Brain. Res. 663:199–205.
Riederer, P., Sofie, E., Rausch, W. D., Schmidt, B., Reynold, G. P., Jellinger, K., and Youdim, M. B. H. 1989. Transition metals, ferritin, glutathione and ascorbic acid in Parkinsonian brain. J. Neurochem. 52:515–520.
Youdim, M. B. H., Ben-Shachar, D., and Riederer, P. 1993. The possible role of iron in the etiopathology of Parkinson's disease. Mov. Disord. 8:1–12.
Nakamura, Y., Takeda, M., Suzuki, H., Hattori, H., Tada, K., Hariguchi, S., Hashimoto, S., and Nishimura, N. 1991. Abnormal distribution of cathepsins in the brain of patients with Alzheimer's disease. Neurosci. Lett. 13:195–198.
Topper, R., Gerhmann, J., Banati, R., Schwarz, M., Block, F., Noth, J., and Kreutzberg, G. W. 1995. Rapid appearance of beta-amyloid precursor protein immunoreactivity in glial cells following excitotoxic brain injury. Acta Neuropathol. Berl. 89:23–28.
Oropeza, R. L., Wekerle, H., and Werb, Z. 1987. Espression of apolipoprotein E by mouse brain astrocytes and its modulation by interferon-gamma. Brain. Res. 410:45–51.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tacconi, M.T. Neuronal Death: Is There a Role for Astrocytes?. Neurochem Res 23, 759–765 (1998). https://doi.org/10.1023/A:1022463527474
Issue Date:
DOI: https://doi.org/10.1023/A:1022463527474