Abstract
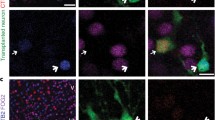
Fetal septal neurons transplanted into the deafferented retrosplenial cortex (RSC) of rats have been shown to reinnervate the host brain and ameliorate spatial memory deficits. In the present study we examined the effects of implanting cholinergic neurons on high affinity choline uptake (HACU) in the denervated RSC and the correlational relationship between this cholinergic parameter and the level of behavioral recovery. Three groups of animals were used: 1) normal control rats (NC), 2) rats with lesions of the fornix and cingulate pathways (FX), and 3) lesioned rats with fetal septal grafts in the RSC (RSCsep-TPL). We found that intra-RSC septal grafts produced significant increases in HACU, and that recovery of HACU was significantly correlated with the improvements in the performance of spatial reference memory, spatial navigation, and spatial working memory tasks. We have also investigated the ability of the host brain to modulate the activity of the implanted neurons. In particular we evaluated the effect of the animals' performance in a 6-arm radial maze task on high affinity choline uptake (HACU). Animals in each of the NC, FX, and RSCsep-TPL groups were randomly assigned one of the following subgroups: 1) rats that performed the maze task before the determination of HACU (BEH), or 2) rats that did not perform the maze task before the determination of HACU (NON-BEH). Significant increases were observed in the NC and RSCsep-TPL groups, but not in the FX animals, indicating that fetal septal grafts in the RSC can become functionally incorporated with the host neural circuitry, and that the activity of the implanted cholinergic neurons can be modulated by the host brain.
Similar content being viewed by others
REFERENCES
Shute, C., and Lewis, P. 1967. The ascending cholinergic reticular system: neocortical, olfactory, and subcortical projections. Brain 90:492–520.
Wenk, H., Bigl, V., and Meyer, U. 1980. Cholinergic projections from magnocellular nuclei of the basal forebrain to cortical areas in rats. Brain Res. Rev. 2:295–316.
Bigl, V., Woolf, N. J., and Butcher, L. L. 1982. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res. Bull. 8:727–749.
Woolf, N. J., and Bucher, L. L. 1982. Cholinergic projections to the basalateral amygdala: a combined Evans Blue and acetylcholinesterase analysis. Brain Res. Bull. 8:751–763.
Mesulam, M. M., Mufson, E. J., Levey, A. I., and Wainer, B. H. 1983. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 214:170–197.
Saper, C. B. 1984 Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J. Comp. Neurol. 222:313–342.
Woolf, N. J., Hernit, M. C., and Butcher, L. L. 1986. Cholinergic and non-cholinergic projections from the rat basal forebrain revealed by combined choline acetyltransferase and phaseolus vulgaris leucoagglutinin immunocytochemistry. Neurosci. Lett. 66:281–286.
Eckensten, F. P., Baughman, R. W., and Quinn, J. 1988. An anatomical study of cholinergic innervation in rat cerebral cortex. Neuroscience 25:457–474.
Senut, M. C., Menetrey, D., and Lamour, Y. 1989. Cholinergic and peptidergic projections from the medial septum and the nucleus of the diagonal band of Broca to dorsal hippocampus, cingulate cortex and olfactory bulb: a combined wheatgerm agglutinin-apohorseradish peroxidase-gold immunohistochemical study. Neuroscience 30:385–403.
Gage, S. L., Keim, S. R., and Low, W. C. 1994. Nerve Cells in the Medial Septal Nucleus and Diagonal Band of Broca Innervate the Retrosplenial Cortex of the Rat via the Fornix Pathway. Exp. Neurol. (submitted).
Li, Y. J., and Low, W. C. 1993. Intra-retrosplenial cortical transplants of cholinergic neurons derived from the septal nucleus fetal rats: pattern of innervation. J. Neural. Transpl. & Plasti. 3:190–191.
Gabriel, M., Foster, K., Orona, E., Saltwick, S. E., and Stanton, M. 1980. Neuronal activity of cingulate cortex, anteroventral thalamus and hippocampal formation in discriminative conditioning: encoding and extraction of the significance of conditional stimuli. Prog. Psychobiol. Physiol. Psychol. 9:125–231.
Destrade, D., and Ott, T. 1982. Is a retrosplenial (cingulate) pathway involved in the mediation of high frequency hippocampal rhythmic slow activity (theta)? Brain Res. 252:29–37.
Berger, T., Weikart, C., Bassett, J., and Orr, W. 1986. Lesions of the retrosplenial cortex produce deficits in reversal learning of the rabbit nictating membrane response: Implications for potential interactions between hippocampal and cerebellar brain systems. Behav. Neurosci. 100:802–809.
Sutherland, R. J., Whishaw, I. Q., and Kolb, B. 1988. Contributions of cingulate cortex to two forms of spatial learning and memory. J. Neurosci. 8:1863–1872.
Markowska, A., Olton, D., Murray, E., and Gaffan, D. 1989. A comparative analysis of the role of fornix and cingulate cortex in memory. Exp. Brain Res. 74:187–201.
Matsunmi, K., Dawashima, T., and Satake, H. 1989. Mode of [14C]-2-deoxy-glucose uptake into retrosplenial cortex and other memory-related structures of the monkey during a delayed response. Brain Res Bulletin. 22:829–838.
Valenstein, E., Boweres, D., Verfaellie, M., Heiman, K. M., Day A., and Watson, R. T. Retrosplenial amnesia. Brain 110:1631–1646.
Li, Y. J., and Low, W. C. 1996. Intra-retrosplenial grafts of fetal cholinergic neurons and the restoration of spatial memory function. Cell Transplantation (in press).
Simon, J. R., and Kuhar, M. 1976. High affinity choline uptake: ionic and energy requirements. J. Neurochem. 27:93–99.
Simon, J. R., Atweh, S., and Kuhar, M. J. 1976. Sodium dependent high affinity choline uptake: a regulatory step in the synthesis of acetylcholine. J. Neurochem. 36:265–257.
Kuhar, M. J., Sethy, V. H., Roth, R. H., and Aghajanian, G. K. 1973. Choline: selective accumulation by central cholinergic neurons. J. Neurochem. 20:581–593.
Kaseda, U., Simon, J. R., and Low, W. C. 1989. Restoration of high affinity choline uptake in the hippocampal formation following septal cell suspension transplants in rats with fimbria-fornix lesions. J. Neurochem. 53:482–488.
Simon, J. R., and Kuhar, M. J. 1975. Impulse-flow regulation of high affinity choline uptake in brain cholinergic nerve terminals. Nature 255:162–163.
Morris, R. G. M. 1981. Spatial localization does not require the presence of local cues. Learning and Motiva 12:239–260.
Morries, R. G. M., Garrud, P., Rawlins, J., and O'Keefe, J. 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683.
Lowry, O. N., Resebrough, N. J., Farr, A. C., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–257.
Sripanickulchain, K., and Wyss, J. M. 1986. Thalamic projections to retrosplenial cortex in the rat. J. Comp. Neurol. 254:143–165.
Tarricone, B. J., Keim, S. R., Simon, J. R., and Low, W. C. 1991. Intrahippocampal transplants of septal cholinergic neurons: high affinity choline uptake and spatial memory function. Brain Res. 548:55–62.
Li, Y. J., Simon, J. R., and Low, W. C. 1992. Intrahippocampal grafts of cholinergic-rich striatal tissue ameliorate spatial memory deficits in rats with fornix lesions. Brain Res. Bull. 29:147–155.
Hudick, J. P., Wajda, I. J., and Lajtha, A. 1976. Isotopic labelling of synaptosomes that accumulate choline and the effect of narcotic drugs. Neurochem. Res. 1:609–625.
Guyenet, P., Lefresne, P., Rossier, J., Beaujouan, J., and Glowinski, J. 1973. Effect of sodium himicholinium-3 and antiparkinson drugs on [14C]acetylcholine synthesis and [3H]choline uptake in rat striatal synptosomes. Brain Res. 62:523–529.
Kuhar, M. J., and Murrin, L. C. 1978. Sodium-dependent high affinity choline uptake. J. Neurochem. 30:15–21.
Dunnett, S. B., Low, W. C., Iversen, S. D., Stemevo, I., and Bjorklund, A. 1982. Septal transplants restore maze learning in rats with fornix-fimbria lesions. Brain Res. 251:335–348.
Low, W. C., Lewis, R. P., Bunch, S. T., Dunnett, S. B., Thomas, S. R. Iversen, S. D., Bjorklund, A., and Stenevi, U. 1982. Function recovery following neural transplantation of embryonic septal nuclei in adult rats with septohippocampal lesions. Nature 300:260–262.
Nilsson, O. G., Shapiro, M. L., Olton, D. S., and Bjorklund, A. 1987. Spatial learning and memory following fimbria-fornix transection and grafting of fetal septal neurons to the hippocampus. Exp. Brain Res. 67:195–215.
Nilsson, O. G., Kalen, P., Rosenbren, E., and Bjorklund, A. 1990. Acetylcholine release from intrahippocampal septal graft is under control of the host brain. Proc. Natl. Acad. Sci. USA. 87:2647–2651.
Buzsaki, G., Gage, F. H., and Bjorklund, A. 1987. Restoration of rhythmic slow activity in the subcortically denervated hippocampus by fetal CNS transplants. Brain Res. 400:334–337.
Wenk, G., Hepler, D., and Olton, D. 1984. Behavior alters the uptake of [3H]choline into acetylcholinergic neurons of the nucleus basalis magnocellularis and medial septal area. Behav. Brain Res. 13:129–138.
Toumane, A., Durkin, T., Marighetto, A., Galey, D., and Jaffard, R. 1988. Differential hippocampal and cortical cholinergic activation during the acquisition, retention, reversal and extinction of a spatial discrimination in an 8-arm radial maze by mice. Behav. Brain Res. 30:225–234.
Toumane, A., Durkin, T., Marighetto, A., and Jaffard, R. 1989. The duration of hippocampal and cortical cholinergic activation induced by spatial discrimination testing of mice in an eight-arm radial maze decrease as a function of acquisition. Behav. Neural Biol. 52:279–284.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, Y.J., Low, W.C. Intra-Retrosplenial Cortical Grafts of Cholinergic Neurons: Functional Incorporation and Restoration of High Affinity Choline Uptake. Neurochem Res 22, 589–595 (1997). https://doi.org/10.1023/A:1022422103674
Issue Date:
DOI: https://doi.org/10.1023/A:1022422103674