Abstract
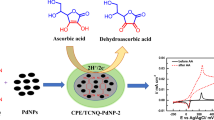
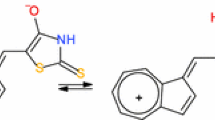
The electrochemical properties of a glassy-carbon electrode coated with a polyvinylpyridine film doped with incorporated cobalt phthalocyanine were studied in a reaction involving a benzoquinone–hydroquinone redox couple. It was found that poly-(2-vinylpyridine) film applied to the electrode and cobalt phthalocyanine deposited onto it or incorporated in the polymeric film exhibited electrocatalytic activity on the oxidation of hydroquinone. Conditions were selected for obtaining a polyvinylpyridine film doped with cobalt phthalocyanine on the electrode surface providing a maximum catalytic effect. The current of the hydroquinone oxidation peak and the current of the reverse benzoquinone reduction peak at the chemically modified electrode were linear functions of their concentrations in the range from 1 × 10–6 to 1 × 10–3 M.
Similar content being viewed by others
REFERENCES
Budnikov, G.K., Maistrenko, V.N., and Murinov, Yu.I., Vol'tamperometriya s modifitsirovannymi i ul'tramikroelektrodami (Voltammetry with Modified Electrodes and Ultramicroelectrodes), Moscow: Nauka, 1994.
Anderson, J.L., Bowden, E.F., and Picput, P.G., Anal. Chem., 1996, vol. 68, no. 12, p. 379.
Labuda, Ya., Zh. Anal. Khim., 1990, vol. 45, no. 3, p. 629.
Tarasevich, M.R. and Radyushkina, K.A., Kataliz i elektrokataliz metalloporfirinami (Catalysis and Electrocatalysis by Metal Porphyrins), Moscow: Nauka, 1982.
Budnikov, G.K., Zh. Anal. Khim., 1996, vol. 51, no. 2, p.156.
Marcor, K.A. and Spiro, T.G., J. Am. Chem. Soc., 1983, vol. 105, p. 5601.
White, B.A. and Murrey, R.W., J. Electroanal. Chem., 1985, vol. 189, p. 345.
Radyushkina, K.A., Tarasevich, M.R., and Radina, M.V., Elektrokhimiya, 1997, vol. 33, no. 1, p. 5.
Efimov, O.N., Vernitskaya, T.V., Danil'chuk, T.N., and Kanevskii, L.S., Elektrokhimiya, 1996, vol. 32, no. 12, p.1486.
Radyushkina, K.A. and Tarasevich, M.R., Elektrokhimiya, 1998, vol. 34, no. 1, p. 102.
Weissberger, A., Proskauer, E.S., Riddick, J.A., and Toops, E.E., Organic Solvents: Physical Properties and Methods of Application, vol. 7 of Technique of Organic Chemistry, Weissberger, A., Ed., New York: Interscience, 1955. Translated under the title Organicheskie rastvoriteli, Moscow: Inostrannaya Literatura, 1958.
Gordon, A.J. and Ford, R.A., The Chemist's Companion: A Handbook of Practical Data, Techniques, and References, New York: Wiley-Interscience, 1972. Translated under the title Sputnik khimika, Moscow: Mir, 1976.
Mairanovskii, S.G., Stradyn', Ya.P., and Bezuglyi, V.D., Polyarografiya v organicheskoi khimii (Polarography in Organic Chemistry), Leningrad: Khimiya, 1975.
Baizer, H., Organic Electrochemistry: An Introduction and a Guide, Baizer, H., Ed., New York: Dekker, 1973. Translated under the title Organicheskaya elektrokhimiya, Moscow: Khimiya, 1988, vol. 2, p. 480.
Heyrovsky, J. and Kuta, J., Zaklady polarografie (Fundamentals of Polarography), Praha: CNTL, 1962. Translated under the title Osnovy polyarografii, Moscow: Mir, 1965.
Budnikov, G.K., Printsipy i primenenie vol'tampernoi ostsillograficheskoi polyarografii (Principles and Application of Voltammetric Oscillographic Polarography), Kazan: Kazansk. Gos. Univ., 1975.
Charykov, A.K., Matematicheskaya obrabotka rezul'tatov khimicheskogo analiza (Mathematical Treatment of the Results of Chemical Analysis), Leningrad: Khimiya, 1984.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shaidarova, L.G., Gedmina, A.V. & Budnikov, G.K. Voltammetry of a Benzoquinone–Hydroquinone Redox Couple at Electrodes Modified with a Polyvinylpyridine Film Doped with Cobalt Phthalocyanine. Journal of Analytical Chemistry 58, 171–175 (2003). https://doi.org/10.1023/A:1022366307246
Issue Date:
DOI: https://doi.org/10.1023/A:1022366307246