Abstract
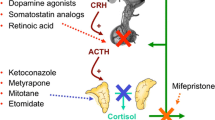
Surgical excision of an ACTH-producing pituitary tumor is the optimal therapy for Cushing's disease. However, medical therapy may have either a primary or adjunctive role if the patient cannot safely undergo surgery, if surgery fails, or if the tumor recurs. When medication is the only therapy, a major disadvantage is the need for lifelong therapy; in general, recurrence follows discontinuation of treatment. These compounds work through three broad mechanisms of action. “Neuromodulatory” compounds modulate corticotropin (ACTH) release from a pituitary tumor, steroidogenesis inhibitors reduce cortisol levels by adrenolytic activity and/or direct enzymatic inhibition and glucocorticoid antagonists block cortisol action at its receptor.
In general, neuromodulatory compounds (bromocriptine, cyproheptidine, somatostatin and valproic acid) are not very effective agents for Cushing's disease. Treatment with a glucocorticoid antagonist and radiation therapy has been reported on a single patient only. Steroidogenesis inhibitors, including mitotane, metyrapone, ketoconazole, and aminoglutethimide, are the agents of choice for medical therapy of Cushing's disease. In general, ketoconazole is the best tolerated of these agents and is effective as monotherapy in about 70% of patients. Mitotane and metyrapone may be effective as single agents, while aminoglutethimide generally must be given in combination. The intravenously-administered etomidate may used when patients cannot take medications by mouth.
Similar content being viewed by others
References
Tomita A, Suzuki S, Hara I, Oiso Y, Mizuno S, Yogo H, Kuwayama A, Kageyama N. Follow-up study on treatment in 27 patients with Cushing's disease: Adrenalectomy, transsphenoidal adenomectomy and medical treatment. Endocrinol Jpn 1981;28:197–205.
Ambrosi B, Gaggini M, Secchi F, Faglia G. Lack of effect of antiserotoninergic and/or dopaminergic treatment in patients with pituitary-dependent Cushing's syndrome. Horm Metab Res 1979;11:318–319.
Krieger DT, Amorosa L, Linick F. Cyproheptadine-induced remission of Cushing's disease. N Engl J Med 1975;293:893–896.
Tyrrell JB, Brooks RM, Forsham PH. More on cyproheptadine. N Engl J Med 1976;295:1137–1138.
Copinschi G, Goldstein J, Virasoro E, Franckson JKM. Lack of effect of cyproheptidine in Cushing's disease and in Nelson's syndrome (abstr). Acta Endocrinol (Copenh) 1977;312:ob-Suppl 130J:90.
Sonino N, Fava GA, Fallo F, Franceschetto A, Belluardo P, Boscaro M. Effect of the serotonin antagonists ritanserin and ketanserin in Cushing's disease. Pituitary 2000;3:55–59.
Miller JW, Crapo L. The medical treatment of Cushing's syndrome. Endocr Rev 1993;14:443–458.
Nussey SS, Price P, Jenkins JS, Altaher AR, Gillham B, Jones MT. The combined use of sodium valproate and metyrapone in the treatment of Cushing's syndrome. Clin Endocrinol (Oxf) 1988;28:373–380.
Stalla GK, Brockmeier SJ, Renner U, Newton C, Buchfelder M, Stalla J, Muller OA. Octreotide exerts different effects in vivo and in vitro in Cushing's disease. Eur J Endocrinol 1994;130:125–131.
Lamberts SW, Uitterlinden P, Klijn JM. The effect of the long-acting somatostatin analogue SMS 201-995 on ACTH secretion in Nelson's syndrome and Cushing's disease. Acta Endocrinol (Copenh) 1989;120:760–766.
Woodhouse NJ, Dagogo-Jack S, Ahmed M, Judzewitsch R. Acute and long-term effects of octreotide in patients with ACTH-dependent Cushing's syndrome. Am J Med 1993;95:305–308.
Vignati F, Loli P. Additive effect of ketoconazole and octreotide in the treatment of severe adrenocorticotropin-dependent hypercortisolism. J Clin Endocrinol Metab 1996;81:2885–2890.
Pivonello R, Faggiano A, Di Salle F, Filippella M, Lombardi G, Colao A. Complete remission of Nelson's syndrome after 1-year treatment with cabergoline. J Endocrinol Invest 1999;22:860–865.
Potts GO, Creange JE, Harding HR, Schane HP. Trilostane, an orally active inhibitor of steroid biosynthesis. Steroids 1978;32:257–267.
Santen RJ, Misbin RI. Aminoglutethimide: Review of pharmacology and clinical use. Pharmacotherapy 1981;1:95–120.
Feldman D. Ketoconazole and other imidazole derivatives as inhibitors of steroidogenesis. Endocr Rev 1986;7:409–420.
Hutter AM, Kayhoe DE. Adrenal cortical carcinoma: Results of treatment with o, p?-DDD in 138 patients. Am J Med 1966;41:581–592.
Gorden P, Becker C, Levey GS, Roth J. Efficacy of aminoglutethimide in the ectopic ACTH syndrome. J Clin Endocrinol 1968;28:921–923.
Verhelst JA, Trainer PJ, Howlett TA, et al. Short-and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol 1991;35:169–178.
Orth DN. Metyrapone is useful only as adjunctive therapy in Cushing's disease (editorial). Ann Intern Med 1978;89:128–130.
Misbin R, Canary J, Willard D. Aminoglutethimide in the treatment of Cushing's syndrome. J Clin Pharmacol 1976;16:645–651.
Zachman M, Gitzelman RP, Zagalak M, Prader A. Effect of aminoglutethimide on urinary cortisol and cortisol metabolites in adolescents with Cushing's syndrome. Clin Endocrinol 1977;7:63–71.
Horky K, Kuchel O, Gregvoria I, Starka L. Qualitative alterations in urinary 17-ketosteroid excretion during aminoglutethimide administration. J Clin Endocrinol Metab 1969;29:297–301.
Hart MM, Swackhamer ES, Straw JA. Studies on the site of action of o, p?-DDD in the dog adrenal cortex: TNPH and corticosteroid precursor stimulation of o, p?-DDD inhibited steroidogenesis. Steroids 1971;17:575–586.
25. Ojima M, Saitoh M, Itoh N, et al. The effects of o, p-DDD on adrenal steroidogenesis and hepatic steroid metabolism. Nipon Naibunpi Gakkai Zasshi Folia Endocrinol Jpn 1985;61:168–178.
Lubitz JA, Freeman L, Okun R. Mitotane use in inoperable adrenal cortical carcinoma. JAMA 1973;223:1109–1112.
Guttierrez ML, Crooke ST. Mitotane (o, p?-DDD) in inoperable adrenocortical carcinoma. Cancer Treat Rev 1980;7:49–55.
Luton JP, Mahoudeau JA, Bouchard P, et al. Treatment of Cushing's disease by o, p?-DDD: Survey of 62 cases. N Engl J Med 1979;300:459–464.
Schteingart DE, Tsao HS, Taylor CI, McKenzie A, Victoria R, Therrien BA. Sustained remission of Cushing's diseaes with mitotane and pituitary irradiation. Ann Intern Med 1980;92:613–619.
Leiba S, Weinstein R, Shindel B, et al. The protracted effect of o, p?-DDD in Cushing's disease and its impact on adrenal morphogenesis of young human embryo. Ann Endocrinol 1989;50:49–53.
Hague RV, May W, Cullen DR. Hepatic microsomal enzyme induction and adrenal crisis due to o, p?-DDD therapy for metastatic adrenocortical carcinoma. Clin Endocrinol 1989;31:51–57.
Bledsoe T, Island DP, Ney RL, Liddle GW. The effect of o, p?-DDD on cortisol and 6 beta hydroxycortisol secretion and metabolism in man. J Clin Endocrinol Metab 1971;24:1303–1311.
Dewis DC, Bullock DE, Earnshaw R, Kelly WF. Experience with trilostane in the treatment of Cushing's syndrome. Clin Endocrinol 1983;18:533–540.
Ross WM, Evered DC, Hunter P, et al. Treatment of Cushing's disease with adrenal blocking drugs and megavoltage therapy to the pituitary. Clin Radiol 1979;30:149–153.
Fishman LM, Liddle GW, Island DP, et al. Effects of aminoglutethimide on adrenal function in man. J Clin Endocrinol Metab 1967;27:481–490.
Thoren M, Adamson U, Sjoberg HE. Aminoglutethimide and metyrapone in the management of Cushing's syndrome. Acta Endocrinol (Copenh) 1985;109:451–457.
Jeffcoate WJ, Rees LH, Tomlin S, et al. Metyrapone in longterm management of Cushing's disease. Br J Med 1977;2:215–217.
Dickstein G, Lahav M, Shen-Orr Z, Edoute Y, Barzilai D. Primary therapy for Cushing's disease with metyrapone. JAMA 1986;255:1167–1169.
Donckier J, Burrin JM, Ramsay ID, Joplin GF. Successful control of Cushing's disease in the elderly with long term metyrapone. Postgrad Med J 1986;62:727–730.
Child DF, Burke CW, Burley DM, et al. Drug control of Cushing's syndrome: Combined aminoglutethimide and metyrapone therapy. Acta Endocrinol (Copenh) 1976;82:330–341.
Schteingart DE, Conn JW. Effects of aminoglutethimide upon adrenal function and cortisol metabolism in Cushing's syndrome. J Clin Endocrinol Metab 1967;27:1657–1666.
Santen RJ, Misbin RI. Aminoglutethimide: Review of pharmacology and clinical use. Pharmacotherapy 1981;1:95–120.
Sonino N. The use of ketoconazole as an inhibitor of steroid production. N Engl J Med 1987;317:812–818.
Loose DS, Kan PB, Hirst MA, Marcus RA, Feldman D. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J Clin Invest 1983;71:1495–1499.
Lamberts SW, Bons EG, Bruining HA, de Jong FH. Differential effects of the imidazole derivatives etomidate, ketoconazole and miconazole and of metyrapone on the secretion of cortisol and its precursors by human adrenocortical cells. J Pharmacol Exp Ther 1987;240:259–264.
Jimenez Reina L, Leal-Cerro A, Garcia J, Garcia-Luna PP, Astorga R, Bernal G. In vitro effects of ketoconazole on corticotrope cell morphology and ACTH secretion of two pituitary adenomas removed from patients with Nelson's syndrome. Acta Endocrinol (Copenh) 1989;121:185–190.
Stalla GK, Stalla J, Huber M, Loeffler JP, Hollt V, vonWerder K, Muller OA. Ketoconazole inhibits corticotropic cell function in vitro. Endocrinology 1988;122:618–623.
Burrin JM, Yeo TH, Ashby MJ, Bloom SR. Effect of ketoconazole on adrenocorticotrophic hormone secretion in vitro and in vivo. J Endocrinol 1986;108:37–41.
Engelhardt D, Weber MM. Therapy of Cushing's syndrome with steroid biosynthesis inhibitors. J Steroid Biochem Mol Biol 1994;49:261–267.
Angeli A, Frairia R. Ketoconazole therapy in Cushing's disease. Lancet 1985;6;1(8432):821.
Boscaro M, Sonino N, Rampazzo A, Mantero F. Response of pituitary-adrenal axis to corticotrophin releasing hormone in patients with Cushing's disease before and after ketoconazole treatment. Clin Endocrinol (Oxf) 1987;27:461–467.
Lewis JH, Hyman JZ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy. Gastroenterology 1984;86:503–513.
Winquist EW, Laskey J, Crump M, Khamsi F, Shepherd FA. Ketoconazole in the management of paraneoplastic Cushing's syndrome secondary to ectopic adrenocorticotropin production. J Clin Oncol 1995;13:157–164.
Schulte HM, Benker G, Reinwein D, et al. Infusion of low dose etomidate: Correction of hypercortisolemia in patients with Cushing's syndrome and dose-response relationship in normal subjects. J Clin Endocrinol Metab 1990;70:1426–1430.
Drake WM, Perry LA, Hinds CJ, et al. Emergency and prolonged use of intravenous etomidate to control hypercortisolemia in a patient with Cushing's syndrome and peritonitis. J Clin Endocrinol Metab 1998;83:3542–3544.
Krakoff J, Koch CA, Calis KA, Alexander RH, Nieman LK. Use of a parenteral propylene glycol-containing etomidate preparation for the long-term management of ectopic Cushing's syndrome. J Clin Endocrinol Metab 2001;86:4104–4108.
Nieman L, Chrousos G, Kellner C, et al. Successful treatment of Cushing's syndrome with the glucocorticoid antagonist RU 486. J Clin Endocrinol Metab 1985;61:536–540.
Chu JW, Matthias DF, Belanoff J, Schatzberg A, Hoffman AR, Feldman D. Successful long-term treatment of refractory Cushing's disease with high-dose mifepristone (RU 486). J Clin Endocrinol Metab 2001;86:3568–3573.
Gaillard RC, Riondel A, Muller AF, et al. RU 486. A steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci USA 1984;81:3879–3882.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nieman, L.K. Medical Therapy of Cushing's Disease. Pituitary 5, 77–82 (2002). https://doi.org/10.1023/A:1022308429992
Issue Date:
DOI: https://doi.org/10.1023/A:1022308429992