Abstract
Activation energy is calculated from a single curve of a derivative of mass loss perturbed by a sinusoidal modulation of a temperature-time relationship. The method is based on a prediction of a hypothetical derivative of mass loss that corresponds to the absence of this modulation (perturbation). Simple considerations show that the unperturbed derivative coincides with the modulated derivative at inflection points of the modulated temperature-time relationship. The ratio of the perturbed and unperturbed derivatives at the points of time corresponding to maxima and minima of the sinusoidal component of the modulated temperature immediately leads to activation energy. Accuracy of the method grows with decreasing in the amplitude of the modulation.
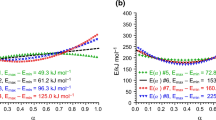
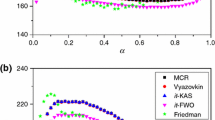
All illustrations are prepared numerically. It makes possible to objectively test the method and to investigate its errors. Two-stage decomposition kinetics with two independent (parallel) reactions is considered as an example. The kinetic parameters are chosen so that the derivative of mass loss would represent two overlapping peaks. The errors are introduced into the modulated derivative by the random-number generator with the normal distribution. Standard deviation for the random allocation of errors is selected with respect to maximum of the derivative. If the maximum of the derivative is observed within the region from 200 to 600°C and the amplitude of the temperature modulation is equal to 5°C, the error in the derivative 0.5% leads to the error in activation energy being equal to 2-6 kJ mol-1. As the derivative vanishes, the error grows and tends to infinity in the regions of the start and end of decomposition. With the absolute error 0.5% evaluations of activation energy are impossible beyond the region from 5 to 95% of mass loss.
Similar content being viewed by others
References
J. Šesták, Thermophysical Properties of Solids, Elsevier, Amsterdam 1984.
A. I. Lesnikovich and S. V. Levchik, J. Thermal Anal., 27 (1983) 89.
A. V. Nikolaev, V. A. Logvimenko and V. M. Gorbachev J. Thermal Anal., 6 (1974) 473.
V. Mamleev, S. Bourbigot, M. Le Bras, S. Duquesne and J. Šesták, Phys. Chem. Chem. Phys., 2 (2000) 4708.
V. Mamleev, S. Bourbigot, M. Le Bras, S. Duquesne and J. Šesták, Phys. Chem. Chem. Phys., 2 (2000) 4796.
V. Mamleev, S. Bourbigot, M. Le Bras, S. Duquesne and J. Šesták, Euroasian Chemico Technological Journal, 2 (2000) 201.
H. H. Horowitz and G. Metzger, Anal. Chem., 35 (1963) 1464.
T. Ozawa, Bull. Chem. Soc. Japan, 38 (1965) 1881.
J. H. Flynn and L. A. Wall, Polym. Lett., 4 (1966) 323.
D. Price, D. Dollimore, N. S. Fatemi and R. Whitehead, Thermochim. Acta, 42 (1980) 323.
C. H. Li, AIChE J., 31 (1985) 1036.
R. K. Agrawal, J. Therm. Anal., 32 (1987) 149.
A. A. Zuru, R. Whitehead and D. L. Griffiths, Thermochim. Acta, 164 (1990) 285.
J. Opfermann, F. Giblin, J. Mayer and E. Kaisersberger, American Laboratory, Febr., 1995, p. 34.
"standard Test Method for Decomposition Kinetics by Thermogravimetry’ ASTM Test method E1641. ASTM Book of Standards 14.02, 1042-1046, American Society for Testing and Materials, 1994.
R. Blaine, American Laboratory, Jan., 1998, p. 21.
S. Vyazovkin, J. Thermal Anal., 49 (1997) 1493.
S. Vyazovkin, J. Comput. Chem., 18 (1997) 393.
S. Vyazovkin, Thermochim. Acta, 355 (2000) 155.
R. L. Blaine and B. K. Hahn, J. Thermal Anal., 54 (1998) 695.
J. H. Flynn, Thermal Analysis, Vol. 2, R. F. Schwenker and P. D. Garn, Eds, Plenum Press, New York, 1969, p. 1111.
G. F. Forsite, M. A. Malcolm and C. B. Moler, Computer Methods for Mathematical Computations, New Jersey: Englewood Cliffs, Prentice-Hall 1977.
R. K. Otnes and L. Enochson, Applied time series analysis, New York, Wiley-Interscience Publication, Wiley&Sons, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mamleev, V., Bourbigot, S. Calculation of activation energies using the sinusoidally modulated temperature. Journal of Thermal Analysis and Calorimetry 70, 565–579 (2002). https://doi.org/10.1023/A:1021697128851
Issue Date:
DOI: https://doi.org/10.1023/A:1021697128851