Abstract
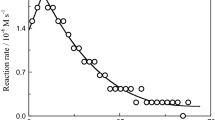
The kinetics and mechanism of chromium(VI) oxidation of L-methionine in acidic medium have been studied spectrophotometrically. The reaction proceeds via the formation of a transient intermediate (λmax = 410–420 nm) which decomposes by a proton catalyzed pathway. The rate law is:
The activation enthalpy and activation entropy for the reaction have been calculated to be ΔH * = 43.85 kJ mol−1, ΔS * = −286.87 JK−1 mol−1. Also values of k 1, k −1 and k 3 were determined: 27.2 × 10−3 M−1 S−1, 1.97 × 10−3 S−1, 7.2 × 10−3 s−1, respectively. The results are compared with those of related studies for reduction of chromate by amino acids.
Similar content being viewed by others
References
H.H. Sky-Peck, Clin. Physiol. Biochim., 4, 99 (1986), H. Babich, M.A. Devanas and G. Stotzky, Environ. Res., 37, 253 (1985).
J. Aiyar, H.J. Berkovits, R.A. Floyd and K.E. Wetterhahn, Chem. Res. Toxicol., 3, 595 (1990), S.C. Rossi and K.E. Wetterhahn, Carcinogenesis, 10, 913 (1989). J. Aiyar, K.M. Borges, R.A. Floyd and K.E. Wetterhahn, Toxicol. Environ. Chem., 22, 135 (1989).
A.M. Fan and I. Harding-Barlow, Adv. Mod. Environ. Toxicol., 11, 87 (1987).
S. Kawanishi, S. Inove and S.J. Sand, Biol. Chem., 261, 5952 (1986).
B.R. Banks and R.T. Cooke, Biochem. Biophys. Res. Commun., 137, 8 (1986).
K. Wetterhahn-Jennette, J. Am. Chem. Soc., 104, 874 (1982).
S.C. Rossi, N. Gorman and K.E. Wetterhahn, Chem. Res. Toxicol., 1, 101 (1988).
D. Ryberg, Alexander, J. Biochem. Pharmacol., 33, 2461 (1984).
A.G. Levis and V. Bianchi, in S. Langard (Ed), Biological and Environmental Aspects of Chromium Elsevier Biomedical Press, Amsterdam, 1982; pp. 171-208.
P. O'Brien, G. Wang and P.B. Wyatt, Polyhedron, 11, 3216 (1992).
R.N. Bose, S. Moghaddas and E. Gelerinter, Inorg. Chem., 31, 1987 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mansour, M.A. Kinetics and mechanism of chromium(VI) oxidation of L-methionine. Transition Metal Chemistry 27, 818–821 (2002). https://doi.org/10.1023/A:1021326228930
Issue Date:
DOI: https://doi.org/10.1023/A:1021326228930