Abstract
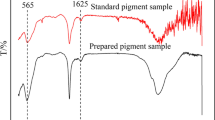
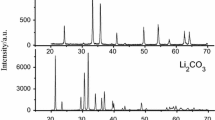
One method of industrial manufacture of red ferric pigments is based on the thermal decomposition of FeSO4⋅7H2O into α-Fe2O3(copperas red). The difficult reproducibility of the color quality of the final pigment is the main problem of this process. One of the factors that can influence the pigment color is contamination by some of the intermediates formed during the transformation process. The identification of two groups of intermediates is the basic result of an extensive laboratory investigation carried out using 57Fe Mössbauer spectroscopy and X-ray powder diffraction (XRD). The first group of intermediates includes sulfato-phases as FeSO4⋅H2O, FeSO4, Fe(OH)SO4, Fe2O(SO4)2, Fe2O(SO4)2⋅xH2O x∈(0,1), and Fe2(SO4)3. Thermally metastable polymorphs of iron(III) oxide, β-Fe2O3, γ-Fe2O3 and ε-Fe2O3, represent the other group. Mössbauer characterization of all intermediate products is given. A significant influence of β-Fe2O3 on the pigment color was found.
Similar content being viewed by others
References
Neto, K. S. and Garg, V. K., J. Inorg. Nucl. Chem. 37 (1975), 2287.
Soroka, P. I., Parkhomenko, V. D., Belous, V. P. and Parkhomenko, N. V., Zh. Prikl. Khim. 53 (1980), 1725.
Bristoti, A., Kunrath, J. I., Viccaro, P. J. and Bergter, L., J. Inorg. Nucl. Chem. 37 (1975), 1149.
Pelovski, Y., Petkova, V. and Nikolov, S., Thermochim. Acta 274 (1996), 273.
Gallagher, P. K., Johnson, D. W. and Schrey, F., J. Am. Ceram. Soc. 53 (1970), 666.
Pelovski, Y. and Petkova, V., J. Therm. Anal. 49 (1997), 1227.
Swamy, M. S. R., Prasad, T. P. and Sant, B. R., J. Therm. Anal. 16 (1979), 471.
Vertes, A. and Zsoldos, B., Acta Chim. Acad. Sci. Hungar. 6 (1970), 261.
Kamel, A. H., Sawires, Z., Khalifa, H., Saleh, S. A. and Abdallah, A. M., J. Appl. Chem. Biotechnol. 22 (1972), 591.
Swamy, M. S. R. and Prasad, T. P., J. Therm. Anal. 20 (1981), 107.
Margulis, E. V., Shokarev, M. M., Savtchenko, L. A., Kopylov, N. I. and Bejsekeeva, L. I., Zh.Neorg. Khim. 16 (1971), 734.
Ismail, H. M., Zaki, M. I., Hussein, A. M. and Magar, M. N., Powder Technol. 63 (1990), 87.
Lorant, B., Z. Analyt. Chem. 219 (1966), 256.
Powder Diffraction File 1997, International Center for Diffraction Data, Pennsylvania, USA.
Zboril, R., Mashlan, M. and Krausova, D., Czech. J. Phys. 51 (2001), 719.
Zboril, R., Mashlan, M., Krausova, D. and Pikal, P., Hyp. Interact. 121–122 (1999), 497.
Zboril, R., Mashlan, M. and Krausova, D., In: M. Miglierini and D. Petridis (eds), MössbauerSpectroscopy in Materials Science, Kluwer Academic Publishers, Dordrecht, 1999, p. 49.
Pascal, C., Pascal, J. L., Favier, F., Elidrissi Moubtassim, M. L. and Payen, C., Chem. Mater. 11 (1999), 141.
Liu, T., Guo, L., Tao, Y., Wang, Y. B. and Wang, W. D., Nanostruct. Mater. 11 (1999), 487.
Iijima, S., Nomura, A., Mizukami, F., Shin, S. and Mizutani, F., J. Radioanal. Nucl. Chem. 239 (1999), 297.
de Bakker, P. M. A., de Grave, E., Vandenberghe, R. E. and Bowen, L. H., Hyp. Interact. 54 (1990), 493.
Tronc, E., Chanéac, C. and Jolivet, J. P., J. Solid State Chem. 139 (1998), 93.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zboril, R., Mashlan, M., Petridis, D. et al. The Role of Intermediates in the Process of Red Ferric Pigment Manufacture from FeSO4⋅7H2O. Hyperfine Interactions 139, 437–445 (2002). https://doi.org/10.1023/A:1021259432720
Issue Date:
DOI: https://doi.org/10.1023/A:1021259432720