Abstract
The kinetics of the reactions of ground state silicon ions Si+(2P) with C2H6, CH3OH, and CH3CH2OH has been investigated using a Selected Ion Flow Drift Tube technique (SIFDT). The reaction rate coefficients and the product distributions have been determined as functions of the reactant ion/reactant neutral average centre-of-mass kinetic energy (E CM).
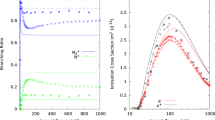
The reactions studied are fast at thermal and near thermal energies and the reaction rate coefficients decrease by nearly one order of magnitude withE CM increasing up to a few eV. It is shown that the energy dependences of the measured reaction rate coefficients can be described by the function of the formk ≈ 1/[1+ (E CM/E CM1)m], whereE CM1 andm are parameters which can be determined from the experimental data. The results are interpreted in terms of a simple model assuming the reactions to proceed via the formation of long-lived complexes. These intermediate complexes decompose with unimolecular rate coefficientsk −1 andk 2 back to the reactants and forward to the products, respectively. The ratiosk −1/k 2 can be approximated for reactions studied by a power law functionk −1/k 2 ∞ (E CM)m.
Similar content being viewed by others
References
Glosík J., Zakouřil P., and Lindinger W.: J. Chem. Phys.103 (1995) 6490.
Glosík J., Zakouřil P., and Lindinger W.: Int. J. Mass Spec. Ion Proc.149/150 (1955) 499.
Glosík J., Zakouřil P., and Lindinger W.: Int. J. Mass. Spec. Ion Proc.145 (1995) 155.
Smith D. and Španěl P.: Acc. Chem. Res.25 (1992) 414.
Smith D.: Chem. Rev.92 (1992) 1473.
Srinivas R., Sülzle D., and Schwarz H.: Chem. Phys. Lett.175 (1990) 575.
Srinivas R., Hrušák J., Sülzle D., Bohme D.K., Schwarz H.: J. Am. Chem. Soc.114 (1992) 2802.
Flores J.R., Largo-Cabrerizo A., and Largo-Cabrerizo J.: J. Mol. Structure (Theochem.)148 (1986) 33.
Largo-Cabrerizo A. and Flores J.R.: Int. J. Quantum Chemistry36 (1989) 241.
Largo A., Barrientos C.: J. Chem. Phys.98 (12994) 3678.
Yamaguchi Y. and Schaefer H.F. III: J. Chem. Phys.102 (1995) 5327.
Lampe F.W.:in Interaction Between Ions and Molecules. NATO ASI (Ed. P. Ausloos), Plenum Press, New York, 1974.
Ferguson E.E., Fahey D.W., Fehsenfeld F.C., and Albritton D.L.: Planet. Space Sci.29 (1981) 307.
Bohme D.K.: Int. J. Mass Spec. Ion Proc.100 (1990) 719.
Bohme D.K.: Advances in Gas Phase Ion Chemistry1 (1992) 225.
Armentrout P.B.:in Structure/Reactivity and Thermochemistry of Ions (Eds. P. Ausloos and S.G. Lias), D. Reidel Publ. Comp., Dordrecht, 1986.
Armentrout P.B.: Advances in Gas Phase Ion Chemistry1 (1992) 225.
Anicich V.G.: J. Phys. Chem. Ref. Data22 (1993) 1469.
Zakouřil P., Glosík J., Skalský V., and Lindinger W.: J. Phys. Chem.99 (1995) 15890.
McDaniel E.W., Čermák V., Dalgarno A., Ferguson E.E., and Friedman L.: Ion-Molecule Reactions, Wiley-Interscience, John Wiley & Sons, New York, 1970.
Smith S.C., McEwan M.J., Giles K., Smith D., and Adams N.G.: Int. J. Mass Spec. Ion Proc.96 (1990) 77.
Albritton D.L.:in Kinetics of ion-molecule reactions (Ed. P. Ausloos), Plenum Publ. Corp., New York, 1979.
Arnold S.T., Dressler R.A., Bastian M.J., Gardner J.A., and Murad E.: J. Chem. Phys.102 (1995) 6110.
Su T. and Bowers M.T.: Int. J. Mass Spec. Ion Phys.12 (1973) 347.
Su T. and Bowers M.T.: J. Chem. Phys.17 (1975) 309.
Futrell J.H.:in Gaseous Ion Chemistry and Mass Spectroscopy (Ed. J.H. Futrell), Wiley & Sons, New York, 1986.
Federer W., Ramler H., Villinger H., and Liindinger W.: Phys. Rev. Lett.54 (1985) 540.
Wannier G.H.: Bell Syst. Tech. J.:32 (1953) 170.
Fahey D.W., Fehsenfeld F.C., Ferguson E.E., and Viehland L.A.: J. Chem. Phys.75 (1981) 669.
Adams N.G. and Smith D.:in Techniques for the study of ion-molecule reactions (Eds. J.M. Farrar and W.H. Saunders, Jr.), John Wiley & Sons, New York, 1988.
Glosík J., Rakshit A.B., Twiddy N.D., Adams N.G., and Smith D.: J. Phys. B11 (1978) 3365.
Wlodek S., Fox A., and Bohme D.K.: J. Am. Chem. Soc.113 (1991) 4461.
Boo B.H. and Armentrout P.B.: J. Am. Chem. Soc.113 (1991) 6421.
Wlodek S., Fox A., and Bohme D.K.: J. Am. Chem. Soc.109 (1987) 6663.
Fox A., Wlodek S., Hopkinson A.C., Lien M.H., Sylvain M., Rodriquez C., and Bohme D.K.: J. Phys. Chem.93 (1989) 1549.
Fox A. and Bohme D.K.: Chem. Phys. Lett.187 (1991) 541.
Glosík J., Smith D., Španěl P., Freysinger W., and Lindinger W.: Int. J. Mass Spec. Ion Proc.129 (1993) 131.
Author information
Authors and Affiliations
Additional information
This work was supported in part by the Fonds zur Förderung der Wissenschaftlichen Forschung under project No. 10014, in part by the Grant Agency of the Czech Republic under project No. 94/0449, and by Charles University, Prague, under project No. GA UK 179/1996.
Rights and permissions
About this article
Cite this article
Glosík, J., Zakouřil, P. & Lindinger, W. Experimental study of the reactions of Si+(2P) ions with several small organic molecules at near thermal energies. Czech J Phys 48, 29–44 (1998). https://doi.org/10.1023/A:1021236212910
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1023/A:1021236212910