Abstract
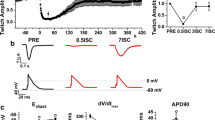
A number of data are consistent with the hypothesis that increases in intracellular Na+ concentration (Na+ i) during ischemia and early reperfusion lead to calcium overload and exacerbation of myocardial injury. However, the mechanisms underlying the increased Na+ i remain unclear. 23Na nuclear magnetic resonance spectroscopy was used to monitor Na+ i in isolated rat hearts perfused with a high concentration of fatty acid as can occur under some pathological conditions. Whole-cell patch-clamp experiments were also performed on isolated cardiomyocytes in order to investigate the role of voltage-gated sodium channels. Na+ i increased to substantially above control levels during no-flow ischemia. The results show that a pharmacological reduction of Na+ i increase by cariporide (1 μmol/L, a Na+/H+ exchange blocker) is not the only protection against ischemia-reperfusion damage, but that such protection may also be brought about by metabolic action aimed at reducing fatty acid utilization by myocardial cells. This action was obtained in the presence of etomoxir (0.1 μmol/L), an inhibitor of carnitine palmitoyltransferase-1 (the key enzyme involved in fatty acid uptake by the mitochondria) which also decreases long-chain acyl carnitine accumulation. The possibility of Na+ channels participating in Na+ i increase as a consequence of alterations in cardiac metabolism was studied in isolated cells. Sustained INa was stimulated by the presence of lysophosphatidylcholine (LPC, 10 μmol/L) whose accumulation during ischemia is, at least partly, dependent on increased long-chain acyl carnitine. Current activation was particularly significant in the range of potentials between −60 and −20 mV. This may have particular relevance in ischemia. The quantity of charge carried by sustained INa was reduced by 24% in the presence of 1 μmol/L cariporide. Therefore, limitation of long-chain fatty acid metabolism, and consequent limitation of ischemia-induced long-chain acyl carnitine accumulation, may contribute to reducing intracellular Na+ increase during ischemia-reperfusion.
Similar content being viewed by others
References
Tani M, Neely JR: Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Circ Res 65: 1045-1056, 1989
Pierce GN, Czubryt MP: The contribution of ionic imbalance to ischemia/reperfusion-induced injury. J Mol Cell Cardiol 27: 53-63, 1995
Murphy E, Cross H, Steenbergen C: Sodium regulation during ischemia vs. reperfusion and its role in injury. Circ Res 84: 1469-1470, 1999
El Banani H, Bernard M, Baetz D, Cabanes E, Cozzone P, Lucien A, Feuvray D: Changes in intracellular sodium and pH during ischaemia-reperfusion are attenuated by trimetazidine. Comparison between low-and zero-flow ischaemia. Cardiovasc Res 47: 688-696, 2000
Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E: Overex-pression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res 83: 1215-1223, 1998
Cross HR, Radda GK, Clarke K: The role of Na+/K+ ATPase activity during low flow ischemia in preventing myocardial injury: A 31P, 23Na and 87Rb NMR spectroscopic study. Magn Res Med 34: 673-685, 1995
Vaughan-Jones RD, Wu ML: Extracellular H+ inactivation of Na+-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol (Lond) 428: 441-466, 1990
Pike MM, Luo CS, Clark MD, Kirk KA, Kitakaze M, Madden MC, Cragoe EJ, Pohost GM: NMR measurements of Na+ and cellular energy in ischemic rat heart: Role of Na+-H+ exchange. Am J Physiol 265: H2017-H2026, 1993
Imahashi K, Hashimoto K, Yamagushi H, Nishimura T, Kusuoka H: Alteration of intracellular Na+ during ischemia in diabetic rat hearts: the role of reduced activity in Na+/H+ exchange against stunning. J Mol Cell Cardiol 30: 509-517, 1998
Park CO, Xiao XH, Allen DG: Changes in intracellular Na+ and pH in rat heart during ischemia: Role of Na+/H+ exchanger. Am J Physiol 276: H1581-H1590, 1999
Le Prigent K, Lagadic-Gossmann D, Mongodin E, Feuvray D: HCO3 −-dependent alkalinizing transporter in adult rat ventricular myocytes: Characterization and modulation. Am J Physiol 273: H2596-H2603, 1997
Khandoudi N, Bernard M, Cozzone P, Feuvray D: Mechanisms of intracellular pH regulation during postischemic reperfusion of diabetic rat hearts. Diabetes 44: 196-202, 1995
Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, Bevensee MO, Boron WF, Bril A: Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res 52: 387-396, 2001
Haigney MCP, Lakatta EG, Stern MD, Silverman HS: Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation 90: 391-399, 1994
Le Grand B, Vie B, Talmant JM, Coraboeuf E, John GW: Alleviation of contractile dysfunction in ischemic hearts by slowly inactivating Na+ current blockers. Am J Physiol 269: H533-H540, 1995
Xiao XH, Allen DG: Role of Na+/H+ exchanger during ischemia and preconditioning in the isolated rat heart. Circ Res 85: 723-730, 1999
Chattou S, Coulombe A, Diacono J, Le Grand B, John G, Feuvray D: Slowly inactivating component of sodium current in ventricular myocytes is decreased by diabetes and partially inhibited by known Na+-H+ exchange blockers. J Mol Cell Cardiol 32: 1181-1192, 2000
Liu B, El Alaoui-Talibi Z, Clanachan AS, Schulz R, Lopaschuk GD: Uncoupling of contractile function from mitochondrial TCA cycle activity and O2 consumption during reperfusion of ischemic rat hearts. Am J Physiol 270: H72-H80, 1996
Feuvray D: Structural, functional, and metabolic correlates in ischemic hearts: Effects of substrates. Am J Physiol 240: H391-H398, 1981
Neely JR, Feuvray D: Metabolic products and myocardial ischemia. Am J Pathol 102: 282-291, 1981
Lewandowski ED: Metabolic mechanisms associated with antianginal therapy. Circ Res 86: 487-489, 2000
Lopaschuk GD, Collins-Nakai R, Olley PM et al.: Plasma fatty acid levels in infants and adults after myocardial ischemia. Am Heart J 128: 61-67, 1994
Scholz W, Albus U, Counillon L, Gögelein H, Lang HJ, Linz W, Weichert A, Schölkens BA: Protective effects of HOE 642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res 29: 260-268, 1995
McHowat J, Creer MH: Lysophosphatidylcholine accumulation in cardiomyocytes requires thrombin activation of Ca2+-independent PLA2. Am J Physiol 272: H1972-H1980, 1997
Pijnappel WWF, Van Der Boogart A, De Beer R, Van Ormondt D: SVD-based quantification of magnetic resonance signals. J Magn Res 97: 122-134, 1992
Vanhamme L, Van Den Boogart A, Van Huffel S: Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35-43, 1997
Antoine S, Lefèvre T, Coraboeuf E, Nottin R, Coulombe A: B-type Ca2+ channels activated by chlorpromazine and free radicals in membrane of human atrial myocytes. J Mol Cell Cardiol 30: 2623-2636, 1998
Kantor PF, Dyck JR, Lopaschuk GD: Fatty acid oxidation in the reperfused ischemic heart. Am J Med Sci 318: 3-14, 1999
Van Emous JG, Nederhoff MGJ, Ruigrok TJC, Van Echteld CJA: The role of the Na+ channel in the accumulation of intracellular Na+ during myocardial ischemia: Consequences for post-ischemic recovery. J Mol Cell Cardiol 29: 85-96, 1997
Davies MJ, Thomas A: Thrombosis and acute coronary artery lesions in sudden cardiac ischemic death. N Engl J Med 310: 1137-1140, 1984
Pinet C, Le Grand B, John GW, Coulombe A: Thrombin is a potent activator of voltage-gated sodium channels in human atrial myocytes. J Mol Cell Cardiol 33: (abstr) A94, 2001
Le Grand B, Marty A, Talmant JM, John GW: HOE 694 affords protection versus veratrine contractures in rat atria by Na+ channel blockade. Fundam Clin Pharmacol 10: 467-473, 1996
Wu J, Corr PB: Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am J Physiol 266: H1034-H1046, 1994
Tanaka M, Gilbert J, Pappano AJ: Inhibition of sodium pump by L-palmitoylcarnitine in single guinea-pig ventricular myocytes. J Mol Cell Cardiol 24: 711-719, 1992
Baetz D, Feuvray D: Modulation of Na+-HCO3 − cotransport (NBC) activity by intracellular signals in adult rat ventricular myocytes. J Mol Cell Cardiol 33: (abstr) A7, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baetz, D., Bernard, M., Pinet, C. et al. Different pathways for sodium entry in cardiac cells during ischemia and early reperfusion. Mol Cell Biochem 242, 115–120 (2003). https://doi.org/10.1023/A:1021197930694
Issue Date:
DOI: https://doi.org/10.1023/A:1021197930694