Abstract
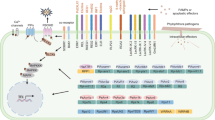
Plants are under continuous threat of infection by pathogens endowed with diverse strategies to colonize their host. Comprehensive biochemical and genetic approaches are now starting to reveal the complex signaling pathways that mediate plant disease resistance. Initiation of defense signaling often involves specific recognition of invading pathogens by the products of specialized host resistance (R) genes. Potential resistance signaling components have been identified by mutational analyses to be required for specific resistance in the model Arabidopsis and some crop species. Strikingly, many of the components share similarity to that of innate immune systems in animals. Evidence is also accumulating that plant pathogens have a number of ways to evade host defenses during the early stages of infection, similar to animal pathogens. These strategies are becoming much better understood in a number of plant–pathogen interactions. In this review, we focus on the current knowledge of host factors that control plant resistance and susceptibility to fungal pathogens. The knowledge accumulated in these studies will serve a fundamental basis for combating diseases in strategic molecular agriculture.
Similar content being viewed by others
References
Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ and Parker JE (1998) Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA 95: 10306–10311.
Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L et al. (1994) Fumonisin-and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol 106: 1085–1093.
Aderem A and Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406: 782–787.
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L et al. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983.
Austin MJ, Muskett P, Kahn K, Feys BJ, Jones JDG and Parker JE (2002) Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295: 2077–2080.
Azevedo C, Sadanandom A, Kitagawa K, Freialdenhoven A, Shirasu K and Schulze-Lefert P (2002) The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295: 2073–2076.
Baker B, Zambryski P, Staskawicz B and Dinesh-Kumar SP (1997) Signalling in plant-microbe interactions. Science 276: 726–733.
Baker SJ, Newton AC, Crabb D, Guy DC, Jeffries RA, Macker-ron DKL et al. (1998) Temporary partial breakdown of mlo-resistance in spring barley by sudden relief of soil water-stress under field conditions: the effects of genetic background and mlo allele. Plant Pathol 47: 401–410.
Blatt MR, Grabov A, Brearley J, Hammond-Kosak K and Jones JDG (1999) K+channels of Cf-9 transgenic tobacco guard cells as targets for Cladosporium fulvum Avr9 elicitor-dependent signal transduction. Plant J 19: 453–462.
Bokoch GM (1994) Regulation of the human neutrophil NADPH oxidase by the Rac GTP-binding proteins. Curr Opin Cell Biol 6: 212–218.
Bowyer P, Clarke BR, Lunness P, Daniels MJ and Osbourn AE (1995) Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science 267: 371–374.
Brandwagt BF, Mesbah LA, Takken FLW, Laurent PL, Kneppers TJA, Hille J et al. (2000) A longevity assurance gene homolog of tomato mediate resistance to Alternaria alternata f.sp. lycopersici toxins and fumonisin B1. Proc Natl Acad Sci USA 97: 4961–4966.
Briggs SP and Johal GS (1994) Patterns of plant host-parasite interactions. Trends Genet 10: 12–16.
Brosch G, Ranson R, Lechner T, Walton JD and Loidl P (1995) Inhibition of maize histone deacetylases by HC toxin, the host-selective toxin of Cochliobolus carbonum. Plant Cell 7: 1941–1950.
Bryan GT, Wu K-S, Farrall L, Jia Y, Hershey HP, McAdams SA et al. (2000) A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12: 2033–2045.
Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A et al. (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88: 695–705.
Cantone FA and Dunkle LD (1991) Reversible effects of inhibitory diffusates from maize inoculated with Cochliobolus carbonum. Physiol Mol Plant Pathol 39: 111–122.
Clarke A, Desikan R, Hurst RD, Hancock JT and Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24: 667–677.
Clough SJ, Fengler KA, Yu I-C, Lippok B, Smith Jr RK and Bent AF (2000) The Arabidopsis thaliana dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 97: 9323–9328.
Dangl JL and Jones JDG (2001) Plant pathogens and integrated defense responses to infection. Nature 441: 826–833.
Delledonne M, Xia YJ, Dixon RA and Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394: 585–588.
Devoto A, Piffanelli P, Nilsson I, Wallin E, Panstruga R, von Heijne G et al. (1999) Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J BiolChem 274: 34993–35004.
Devoto A, Hartmann HA, Piffanelli P, Elliott C, Simmons C, Taramino G et al. (2002) Molecular phylogeny and evolution of the plant-specific seven transmembrane MLO family. J Mol Evol (in press).
Dietrich RA, Richberg MH, Schmidt R, Dean C and Dangl JL (1997) A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88: 685–694.
Doke N (1975) Prevention of the hypersensitive reaction of potato cells to infection with an incomatible race of Phytophthora in-festans by constituents of the zoospores. Physiol Plant Pathol 7: 1–7.
Doke N (1985) NADPH-dependent O –2 generation in membrane fraction isolated from wounded potato tubers inoculated with Phytophthora infestans. Physiol Plant Pathol 27: 311–322.
Durner J, Wendehenne D and Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP ribose. Proc Natl Acad Sci USA 95: 10328–10333.
Durrant WE, Rowland O, Piedras P, Hammond-Kosack KE and Jones JD (2000) cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell 12: 963–977.
Ellis JG, Lawrence GJ, Luck JE and Dodds PN (1999) Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11: 459–506.
Eulgen T, Rushton PJ, Schmelzer E, Hahlbrock K and Somssich IE (1999) Early nuclear events in plant defense signalling: rapid gene activation by WRKY transcription factors. EMBO J 18: 4689–4699.
Falk A, Feys BJ, Frost LN, Jones JDG, Daniels MJ and Parker JE (1999) EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA 96: 3292–3297.
Feys BJ and Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16: 449–455.
Feys BJ, Moisan LJ, Newman M-A and Parker JE (2001) Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20: 5400–5411.
Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopath 9: 275–296.
Freialdenhoven A, Scherag B, Hollricher K, Collinge DB, Thordal-Christensen H and Schulze-Lefert P (1994) Nar-1 and Nar-2, two loci required for Mla 12-specified race-specific resistance to powdery mildew in barley. Plant Cell 6: 983–994.
Freialdenhoven A, Peterhänsel C, Kurth J, Kreuzaler F and Schulze-Lefert P (1996) Identification of genes required for the function of non-race-specific mlo resistance to powdery mildew in barley. Plant Cell 8: 5–14.
Frye CA and Innes RW (1998) An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell 10: 947–956.
Frye CA, Tang D and Innes RW (2001) Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA 98: 373–378.
Gilchrist DG (1998) Programmed cell death in plant disease: the purpose and promise of cellular suicide. Annu Rev Phytopath 36: 393–414.
Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR et al. (1997) Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146: 381–392.
Glazebrook J (2001) Genes controlling expression of defense responses in Arabidopsis. Curr Opin Plant Biol 4: 301–308.
Halterman D, Zhou F, Wei F, Wise RP and Schulze-Lefert P (2001) The MLA6 coiled-coil, NBS-LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f.sp. hordei in barley and wheat. Plant J 25: 335–348.
Hammond-Kosack KE, Tang SJ, Harrison K and Jones JDG (1998) The tomato Cf-9 disease resistance gene functions in tobacco and potato to confer responsiveness to the fungal avirulence gene product Avr9. Plant Cell 10: 1251–1266.
Hückelhoven R, Fodor J, Preis C and Kogel K-H (1999) Hyper-sensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not salicylic acid accumulation. Plant Physiol 119: 1251–1260.
Initiative TAG (2000) Analysis of the genome of the flowering plant Arabidopsis thaliana. Nature 408: 796–815.
Jambunathan N, Siani JM and McNellis TW (2001) A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 13: 2225–2240.
Jarosch B, Kogel K-H and Schaffrath U (1999) The ambivalence of the barley Mlo locus: mutations conferring resistance against powdery mildew (Blumeria graminis f.sp. hordei) enhance susceptibility to the rice blast fungus Magnaporthe grisea. Mol Plant-Microbe Interact 12: 508–514.
Jia Y, McAdams SA, Bryan GT, Hershery HP and Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19: 4004–4014.
Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE et al. (1999) Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA 96: 13583–13588.
Johal GS and Briggs SP (1992) Reductase activity encoded by the HM1 disease resistance gene in maize. Science 258: 985–987.
Jørgensen JH (1977) Spectrum of resistance conferred by ML-O powdery mildew resistance genes in barley. Euphytica 26: 55–62.
Jørgensen JH (1988) Genetic-analysis of barley mutants with modification of powdery mildew resistance genes Ml-a12. Genome 30: 30–132.
Kachroo P, Shanklin J, Shah J, Whittle EJ and Klessig DF (2001) A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA 98: 9448–9453.
Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H et al. (1999) The small GTP-binding protein Rac is a regulator of cell death in plants. Proc Natl Acad Sci USA 96: 10922–10926.
Keen NT (1990) Gene-for-gene complementarity in plant-pathogen interactions. Annu Rev Genet 24: 447–463.
Keller T, Damude HG, Werner D, Doerner P, Dixon RA and Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca 2+ binding motifs. Plant Cell 10: 255–266.
Kiba A, Toyoda K, Ichinose Y, Yamada T and Shiraishi T (1995) Specific inhibition of cell wall-bound ATPases by fungal suppressor from Mycosphaerella pinodes. Plant Cell Physiol 36: 809–817.
Kieber JJ, Rothenberg M, Roman G, Feldmann KA and Ecker JR (1993) CTR1, a negative regulator of the ethylene response path-way in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441.
Kim MC, Panstruga R, Elliott C, Müller J, Devoto A, Yoon HW et al. (2002) Calmodulin interacts with MLO protein to regulate defence against mildew in barley. Nature 416: 447–450.
Kitagawa K, Skowyra D, Elledge SJ, Harper JWand Heiter P (1999) SGT1 encodes an essential components of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin complex. Mol Cell 4: 21–33.
Koga H, Bushnell WR and Zeyen RJ (1990) Specificity of cell type and timing of events associated with papilla formation and the hypersensitive reaction in leaves of Hordeum vulgare attacked by Erysiphe graminis f.sp. hordei. Can J Bot 68: 2344–2352.
Kumar J, Hückelhoven R, Beckhove U, Nagarajan S and Kogel K-H (2000) A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (Teleo-morph: Cochliobolus sativus) and its toxins. Phytopathology 91: 127–133.
Lamb C and Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275.
Leckie F, Mattei B, Capodicasa C, Hemming A, Nuss L, Aracri B et al. (1999) The specificity of polygalacturonase-inhibiting protein (PGIP): a single amino acid substitution in the solvent-exposed â-strand/â-turn region of the leucine-rich repeats (LRRs) confers a new recognition capability. EMBO J 18: 2352–2363.
Leister RT and Katagiri F (2000) A resistance gene product of the nucleotide binding site-leucine rich repeats class can form a complex with bacterial avirulence proteins in vitro. Plant J 22: 345–354.
Ligterink W, Kroj T, zur Nieden U, Hirt H and Scheel D (1997) Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276: 2054–2057.
Martin-Hernandez AM, Dufresne M, Hugouvieux V, Melton R and Osbourn A (2000) Effects of targeted replacement of the tomatinase gene on the interaction of Septoria lycopersici with tomato plants. Mol Plant-Microbe Interact 13: 1301–1311.
Melton RE, Flegg LM, Brown JK, Oliver RP, Daniels MJ and Osbourn AE (1998) Heterologous expression of Septoria lycopersici tomatinase in Cladosporium fulvum: effects on compatible and incompatible interactions with tomato seedlings. Mol Plant-Microbe Interact 11: 228–236.
Moerschbacher BM, Mierau M, Graeßner B, Noll U and Mort AJ (1999) Small oligomers of galacturonic acid are endogenous suppressors of disease resistance reactions in wheat leaves. J ExpBot 50: 605–612.
Morrissey JP and Osbourn AE (1999) Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Revs 63: 708–724.
Muskett PJ, Kahn K, Austin MJm Moisan L, Sadanandom A, Shirasu K, Jones JDG et al. (2002) Arabidopsis RAR1 exerts rate-limiting control of R gene-mediated defences against multiple pathogens. Plant Cell 14: 979–992.
Navarre DA and Wolpert TJ (1999) Victorin induction of an apoptotic/senescence-like response in oats. Plant Cell 11: 273–249.
Nühse TS, Peck SC, Hirt H and Boller T (2000) Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J BiolChem 275: 7521–7226.
Osbourn AE (1996) Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8: 1821–1831.
Papadopoulou K, Melton RE, Leggett M, Daniels MJ and Osbourn AE (1999) Compromised disease resistance in saponin-deficient plant. Proc Natl Acad Sci USA 96: 12923–12928.
Parker JE, Holub EB, Frost LN, Falk A, Gunn ND and Daniels MJ (1996) Characterization of eds1, a mutation in Arabidopsis supressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8: 2033–2046.
Parniske M, Hammond-Kosack KE, Golstein C, Thomas CM, Jones DA, Harrison K et al. (1997) Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf-4/9 locus of tomato. Cell 91: 821–832.
Peterhänsel C, Freialdenhoven A, Kurth J, Kolsch R and Schulze-Lefert P (1997) Interaction analyses of genes required for resistance responses to powdery mildew in barley reveal distinct pathways leading to cell death. Plant Cell 9: 1397–1409.
Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B et al. (2000) Arabidopisis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120.
Pickart CM (2000) Ubiquitin in chains. Trends Biochem Sci 25: 544–548.
Piedras P, Hammond-Kosack KE, Harrison K and Jones JDG (1998) Rapid, Cf-9 and Avr9 dependent production of active oxygen species in tobacco suspension cultures. Mol Plant-Microbe Interact 11: 1155–1166.
Piffanelli P, Zhou F, Casais C, Orme J, Schaffrath U, Collins NC et al. (2002) The barley MLO modulator of defence and cell death is responsive to biotic and abiotic stress stimuli. Plant Phys 129: 1076–1085.
Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H and Jones JD (1999) Rapid Avr9-and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11: 273–287.
Romeis T, Piedras P and Jones JD (2000) Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12: 803–816.
Romeis T, Ludwig AA, Martin R and Jones JD (2001) Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J 20: 5556–5567.
Salmeron JM, Oldroyd GED, Rommens CMT, Scofield SR, Kim HS, Lavelle DT et al. (1996) Tomato Prf is a member of the leucine-rich repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 86: 123–133.
Scofield SR, Tobias CM, Rathjen JP, Chang JH, Lavelle DT, Michelmore RW et al. (1996) Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274: 2063–2065.
Shiraishi T, Saitoh K, Kim H-M, Kato T, Tahara M, Oku H et al. (1992) Two suppressors, supprescins A and B, secreted by a pea pathogen, Mycosphaerella pinodes. Plant Cell Physiol 33: 663–667.
Shiraishi T, Yamada T, Ichinose Y, Kiba A and Toyoda K (1997) The role of suppressor in determining host-parasite specificities in plant cells. Int Rev Cytology 172: 55–93.
Shirasu K, Lahaye T, Tan M-W, Zhou F, Azevedo C and Schulze-Lefert P (1999a) A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans. Cell 99: 355–366.
Shirasu K, Nielsen KA, Piffanelli P, Oliver R and Schulze-Lefert P (1999b) Cell-autonomous complementation of mlo resistance using a transient expression system in barley. Plant J 17: 293–299.
Shirasu K and Schulze-Lefert P (2000) Regulators of cell death in disease resistance. Plant Mol Biol 44: 371–385.
Smart MG, Aist JR and Israel HW (1985) Structure and function of wall appositions. 1. General histochemistry of papillae in barley coleoptiles attacked by Erysiphe graminis f.sp. hordei. Can J Bot 64: 793–801.
Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T et al. (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270: 1804–1806.
Staskawicz BJ, Mudgett MB, Dangl JL and Galan JE (2001) Common and contrasting themes of plant and animal diseases. Science 292: 2285–2289.
Stolzenburg MC, Aist JR and Israel HW (1984) The role of papillae in resistance to powdery mildew conditioned by the ml-o gene in barley. I. Correlative evidence. Physiol Plant Pathol 25: 337–346.
Tang XY, Frederick RD, Zhou JM, Halterman DA, Jia YL and Martin GB (1996) Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274: 2060–2063.
Tao Y, Yuan F, Leister RT, Ausubel FM and Katagiri F (2000) Mutational analysis of the Arabidopsis nucleotide binding siteleucine-rich repeat resistance gene RPS2. Plant Cell 12: 2541–2554.
Tör M, Gordon P, Cuzick A, Eulgem T, Sinapidou E, Mert-Turk F et al. (2002) Arabidopsis SGT1b is required for defense signaling conferred by several downy mildew resistance genes. Plant Cell 14: 993–1003.
Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes R and Dangl JL (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis and their relative contributions are dependent on the R gene assayed. Plant Cell 14: 1005–1015.
Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE and Jones JDG (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 14: 365–370.
Toyoda K, Shiraishi T, Yamada T, Ichinose Y and Oku H (1993) Rapid changes in polyphosphoinositide metabolism in pea in response to fungal signals. Plant Cell Physiol 34: 729–735.
von Röpenack E, Parr A and Schulze-Lefert P (1998) Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J Biol Chem 273: 9013–9022.
Walton JD and Panaccione DG (1993) Host-selective toxins and disease specificity: perspectives and progress. Annu Rev Phytopathol 31: 275–303.
Walton JD (1996) Host-selective toxins: agents of compatibility. Plant Cell 8: 1723–1733.
Wang H, Jones C, Ciacci-Zanella J, Holt T and Gilchrist DG (1996) Fumonisins and Alternaria alternata toxins: sphinganine analog mycotoxins induce apoptosis in monkey kidney cells. Proc Natl Acad Sci USA 93: 3461–3465.
Wolpert TJ, Navarre DA, Moore DL and Macko V (1994) Identification of the 100-kD victorin binding protein from oats. Plant Cell 6: 1145–1155.
Wolter M, Hollricher K, Salamini F and Schulze-Lefert P (1993) The mlo resistance alleles to powdery mildew infection in barley trigger a developmentally controlled defense mimic phenotype. Mol Gen Genet 239: 122–128.
Yamada T, Hashimoto H, Shiraishi T and Oku H (1989) Suppression of pisatin, phenylalanine ammonia-lyase mRNA, and chalcone synthase mRNA by a putative pathogenicity factor from the fungus Mycosphaerella pinodes. Mol Plant-Microbe Interact 2: 256–261.
Yang K-Y, Liu Y and Zhang S (2001) Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 98: 741–746.
Yao N, Tada Y, Park P, Nakayashiki H, Tosa Y and Mayama S (2001) Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J 28: 13–26.
Yoshioka H, Shiraishi T, Yamada T, Ichinose Y and Oku H (1990) Suppression of pisatin production and ATPase activity in pea plasma membranes by orthovanadate, verapamil and a suppressor from Mycosphaerella pinodes. Plant Cell Physiol 31: 1139–1146.
Yoshioka H, Sugie K, Park H-J, Maeda H, Tsuda N, Kawakita K et al. (2001) Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol Plant-Microbe Interact 14: 725–736.
Zhang S and Klessig DF (1998) The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc Natl Acad Sci USA 95: 7225–7230.
Zhou F, Kurth J, Wei F, Elliott C, Valé G, Yahiaoui N et al. (2001) Cell-autonomous expression of barley Mla1 confers race specific resistance to the powdery mildew fungus via a Rar1-independent signaling pathway. Plant Cell 13: 337–350.
Zipperlen P, Fraser AG, Kamath RS, Martinez-Campos M and Ahringer J (2001) Roles for 147 embryonic lethal genes on C. elegans chromosome I identified by RNA interference and video microscopy. EMBO J 20: 3984–3992.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toyoda, K., Collins, N.C., Takahashi, A. et al. Resistance and Susceptibility of Plants to Fungal Pathogens. Transgenic Res 11, 567–582 (2002). https://doi.org/10.1023/A:1021182111770
Issue Date:
DOI: https://doi.org/10.1023/A:1021182111770