Abstract
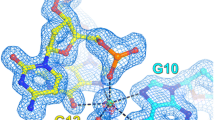
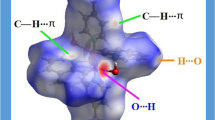
The direct and indirect roles of the C2- and C8-bonded water molecules (C–H ⋅ ⋅ ⋅ OW) in the stabilization of unusual inosine monophosphate containing nucleotides have been observed in their highly hydrated and amino acid cocrystals, which have been discussed in this work for the first time. The intermolecular H-bonding of WR (the oxygen of ribose 2′- and 3′-hydroxyls bonded water molecule, O2′ ⋅ ⋅ ⋅ WR ⋅ ⋅ ⋅ O3′) with the C2–H bonded OW's (OW ⋅ ⋅ ⋅ WR ≈ 2.43–2.78 Å) can influence the ribose and thus the nucleotide stability, whereas the water molecule (OW) of C8–H ⋅ ⋅ ⋅ OW can participate in the base stability by sandwiching the stacked purines through N7 ⋅ ⋅ ⋅ OW ⋅ ⋅ ⋅ N7 intermolecular interactions, with N7 ⋅ ⋅ ⋅ OW ≈ 2.63–2.75 Å. However, in some cases the water oxygen (OW) of C8–H can get intermolecularly H-bonded to water molecular oxygen WN (with OW ⋅ ⋅ ⋅ WN ≈ 2.57–2.85 Å), which has previously stabilized the nucleotide single-strand through intermolecular stacking N7 ⋅ ⋅ ⋅ WN ⋅ ⋅ ⋅ N7 interaction (N7 ⋅ ⋅ ⋅ WN ≈ 2.56–2.63 Å). The conjugal survival of the H-bonded nucleotide single-strand with the water-helix thus forming the duplex and its stabilization through the C–H ⋅ ⋅ ⋅ OW mediated water molecular (OW) cooperative H-bonding network, specially with the ribose and the base units, in the crystals could favor the support of single-stranding potentiality and thus the stability of the purine-rich RNAs or the small unusual nucleotides in the aquated environment besides the other interactive factors.
Similar content being viewed by others
References
Steiner, T.; Saenger, W. J. Am. Chem. Soc. 1993, 115, 4540–4547.
Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, 1992.
Leonard, G.A.; McAuley-Hecht, K.; Brown, T.; Hunter, W.N. Acta Crystallogr., Sect. D 1995, 51, 136–139.
Mayer-Jung, C.; Moras, D.; Timsit, Y. J. Mol. Biol. 1997, 270(3), 328–335.
Aymami, J.; Nunn, C.M.; Neidle, S. Nucleic Acids Res. 1999, 27(13), 2691–2698.
Shi, K.; Biswas, R.; Mitra, S.N.; Sundaralingam, M. J. Mol. Biol. 2000, 299(1), 113–122.
Ghosh, A.; Bansal, M. J. Mol. Biol. 1999, 294(5), 1149–1158.
Ghosh, A.; Bansal, M. Acta Crystallogr., Sect. D 1999, 55(12), 2005–2012.
Desiraju, G.R. Acc. Chem. Res. 1991, 24, 290–296.
Bera, A.K.; Mukhopadhyay, B.P.; Ghosh. S.; Bhattacharya, S.; Chakraborty, S.; Banerjee, A. J. Chem. Crystallogr. 1999, 29(5), 531–540.
Bera, A.K.; Mukhopadhyay, B.P.; Pal, A.K.; Haldar, U.; Bhattacharya, S.; Banerjee, A. J. Chem. Crystallogr. 1998, 28(7), 509–516.
Mukhopadhyay, B.P.; Ghosh, S.; Banerjee, A. J. Chem. Crystallogr. 1995, 25, 477–485.
Bhattacharya, S.; Bera, A.K.; Mukhopadhyay, B.P.; Ghosh., S.; Chakraborty, S.; Pal, A.; Banerjee, A. J. Chem. Crystallogr. 2000, 30(10), 655–663.
Rao, S. T.; Sundralingam, M. J. Am. Chem. Soc. 1969, 91, 1210–1217.
Sriram, M.; Liaw, Y.-C.; Gao, Y.-G.; Wang, A.H.-J. Acta Crystallogr., Sect. C 1991, 47, 507–510.
Nagashima, N.; Wakabayashi, K.; Matzuzaki, T.; Iitaka, Y. Acta Crystallogr., Sect. B 1974, 30, 320–326.
Gabe, E.J.; Le Page, Y.; Charland, J.-P.; Lee, F.L; White, P.S. J. Appl. Crystallogr. 1989, 22, 384–387.
InsightII, version 95.0; Biosym Technologies: SanDiego, CA, 1995.
Kielkopf, C.L.; White, S.; Szewczyk, J.W.; Turner, J.M.; Baird, E.E.; Dervan, P.B.; Rees, D.C. Science 1998, 282(5386), 111–115.
Steiner, T. Acta Crystallogr., Sect. D 1995, 51, 93–97.
Krishnan, R.; Seshadri, T.P.; Viswamitra, M.A. Nucleic Acid Res. 1991, 19(2), 379–384.
Krishnan, R.; Seshadri, T.P. J. Mol. Struct. 2000, 522, 135–143.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bera, A.K., Banerjee, A. & Mukhopadhyay, B.P. Unusual nucleotide stability through C–H ⋅ ⋅ ⋅ water interaction in crystal: Recognition of nucleotide-water duplex-helix. Journal of Chemical Crystallography 32, 531–536 (2002). https://doi.org/10.1023/A:1021113330458
Issue Date:
DOI: https://doi.org/10.1023/A:1021113330458