Abstract
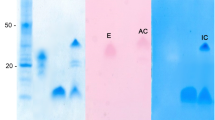
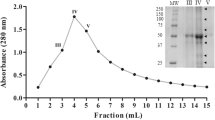
Protein kinase CK2 purified from the yeast Yarrowia lipolytica was used to phosphorylate soybean β-conglycinin α subunit. CK2 is known to phosphorylate serines and threonines in the consensus sequence Ser/Thr-X-X-Glu/Asp/SerP/TyrP. β-Conglycinin α subunit (68 kDa) presents seven consensus sequences, but only 0.5–1 mol P/mol α subunit was incorporated by CK2. [32P]Phosphorylated β-conglycinin α subunit was cleaved either by cyanogen bromide or by trypsin. 32P was incorporated into the largest cyanogen bromide fragment only (50 kDa, N-terminal) and only two radiolabeled zones were detected after HPLC of the trypsic digest. The corresponding phosphorylated zones were collected and further analyzed by RP-HPLC coupled to electrospray ionization mass spectrometry (LC-ESMS). Two phosphorylated sites, Ser 75 and Ser 117, were determined after MS-MS analysis of three phosphopeptides identified as 70–89, 116–126, and 116–127 sequences. Over the seven consensus sequences of β-conglycinin α subunit, Ser 75 is the only one which was phosphorylated. Ser 117 was phosphorylated although it is not an expected phosphorylation site according to the canonical consensus sequence criteria as there is no acidic determinant at the +3 position. Both Ser 75 and Ser 117 are located inside very acidic sequences, by contrast with the other unphosphorylated potential sites.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ralet, MC., Fouques, D., Leonil, J. et al. Soybean β-Conglycinin α Subunit is Phosphorylated on Two Distinct Serines by Protein Kinase CK2 In Vitro. J Protein Chem 18, 315–323 (1999). https://doi.org/10.1023/A:1021091413084
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1021091413084