Abstract
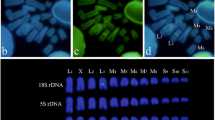
Extensive variation in the size of the short (heterochromatic) arm of chromosome 14 was found in the wasp Trypoxylon (Trypargilum) albitarse. Ten different variants were differentiated by size and C-banding pattern. Fluorescent in-situ hybridization (FISH) revealed that ribosomal DNA in this species is clustered in the darkly C-banded parts of the heterochromatic short arm of chromosome 14. On this basis, we got an indirect estimate of the amount of rDNA from the area of these dark C-bands. The significant absence in males of the three chromosome variants with lower amounts of rDNA indicates that these three variants are lethal in this sex, and suggests the existence of a threshold marking the minimum amount of rDNA which is tolerable in haploidy. This implies about 4% genetic load in the population caused by variation in rDNA amount.
Similar content being viewed by others
References
Araujo SMSR, Pompolo SG, Dergam JAS, Campos LAO (2000) The B chromosome system of Trypoxylon (Trypargilum) albitarse (Hymenoptera, Sphecidae) I. Banding analysis. Cytobios 101: 7-13.
Araújo SMSR, Pompolo SG, Perfectti F, Camacho JPM (2001) Integration of a B chromosomes into the A genome of a wasp. Proc R Soc Lond B 268: 1127-1131.
Bickham JW, Rogers DS (1985) Structure and variation of the nucleolus organizer regions in turtles. Genetica 67: 171-184.
Bohart RM, Menke AS (1976) Sphecidae Wasps of the World: A Generic Revision. Berkeley: University California Press.
Butler DK, Metzenberg RL (1990) Expansion and contraction of the nucleolus organizer region of Neurospora: changes originate in both proximal and distal segments. Genetics 126: 325-333.
Castro J, Rodríguez S, Arias J, Sánchez L, Martínez P (1994) A population analysis of Robertsonian and Ag-NOR polymorphism in brown trout (Salmo trutta). Theor Appl Genet 89: 105-111.
Castro J, Sánchez L, Martínez P (1998) Analysis of the inheritance of NOR size variants in brown trout (Salmo trutta). J Hered 89: 264-266.
Castro J, Rodríguez S, Pardo BG, Sánchez L, Martínez P (2001) Population analysis of an unusual NOR-site polymorphism in brown trout (Salmo trutta L.) Heredity 86: 291-302.
Chindamporn A, Iwaguchi S, Nakagawa Y, Homma M, Tanaka K (1993) Clonal size-variation of rDNA cluster region on chromosome XII of Sacharomyces cerevisiae. J Gen Microbiol 139: 1409-1415.
Ferro DAM, Néo DM, Moreira-Filho O, Bertollo LAC (2001) Nucleolar organizing regions, 18S and 5S rDNA in Astyanax scabripinnis (Pisces, Characidae): populations distribution and functional diversity. Genetica 110: 55-62.
Gatti M, Pimpinelli S (1992) Functional elements in Drosophila melanogaster heterochromatin. Annu Rev Genet 26: 239-275.
Hsu TC, Sperito SE, Pardue ML (1975) Distribution of 18-28S ribosomal genes in mammalian genomes. Chromosoma 53: 25-36.
Imai HT (1991) Mutability of constitutive heterochromatin (C-bands) during eukaryotic chromosomal evolution and their cytological meaning. Jpn J Genet 66: 635-661.
Imai HT, Taylor RW, Crosland MWJ, Crozier RH (1988) Modes of spontaneous chromosome mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Jpn J Genet 63: 113-125.
Karpen GH, Schaefer JE, Laird CD (1988) A Drosophila rRNA gene located in euchromatin is active in transcription and nucleolus formation. Genes Dev 2: 1745-1763.
King M, Contreras N, Honeycutt RL (1990) Variation within and between nucleolar organizer regions in Australian hylid frogs (Anura) shown by 18S+28S in situ hybridisation. Genetica 80: 17-29.
Long EO, Dawid IB (1980) Repeated genes in eukaryotes. Annu Rev Biochem 49: 727-764.
López-León MD, Cabrero J, Camacho JPM (1999) Unusually high amount of inactive ribosomal DNA in the grasshopper Stauroderus scalaris. Chromosome Res 7: 83-88.
Lyckegaard EMS, Clark AG (1991) Evolution of ribosomal RNA gene copy number on the sex chromosomes of Drosophila melanogaster. Mol Biol Evol 8: 458-474.
Macgregor HC, Vlad M, Barnett L (1977) An investigation of some problems concerning nucleolus organizers in salamanders. Chromosoma 59: 283-299.
Martínez P, Viñas A, Bouza C, Castro J, Sánchez L (1993) Quantitative analysis of the variability of nucleolar organizer regions in Salmo trutta. Genome 36: 1119-1123.
Miller OJ (1981) Nucleolar organisers in mammalian cells. Chromosomes Today 7: 64-73.
Miller L, Brown DD (1969) Variation in the activity of nucleolar organizer and their ribosomal gene content. Chromosoma 28: 430-444.
Miller L, Gurdon JB (1970) Mutations affecting the size of the nucleolus in Xenopus laevis. Nature 227: 1108-1110.
Moreira-Filho O, Bertollo LAC, Galetti PM (1984) Structure and variability of nucleolar organizer regions in Parodontidae fish. Can J Genet Cytol. 26: 564-568.
Phillips RB, Pleyte KA, Hartley SE (1988) Stock-specific differences in the number of chromosome positions of the nucleolar organizer regions in artic char (Salvelinus alpinus). Cytogenet Cell Genet 48: 9-12.
Pompolo SG, Takahashi CS (1990) Chromosome number and C-banding in two wasp species of the genus Polistes (Hymenopera, Polistinae, Polistini). Insec Sociaux 37: 251-257.
Roiha H, Miller JR, Woods LE, Glover DM (1981) Arrangements and rearrangements of sequences flanking the two types of rDNA insertion in D. melanogaster. Nature 290: 749-753.
Russell PJ, Roland KD (1986) Magnification of rDNA gene number in a Neurospora crassa strain with partial deletion of the nucleolus organizer. Chromosoma 93: 333-340.
Sánchez L, Martínez P, Viñas A, Bouza C (1990) Analysis of the structure and variability of nucleolar organizer regions of Salmo trutta by C-, Ag-, and restriction endonuclease banding. Cytogenet Cell Genet 54: 6-9.
Schmid M (1982) Chromosome banding in Amphibia. VII. Analysis of the structure and variability of NORs in Anura. Chromosoma 87: 327-344.
Schneider S, Roessli D, Excoffier L (2000) Arlequin ver. 2.000: A software for population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland.
Schubert I, Wobus U (1985) In situ hybridisation confirms jumping nucleolus organizing region in Allium. Chromosoma 92: 143-148.
Strauss SH, Tsai CH (1988) Ribosomal gene number variability in Douglas-fir. J Hered 79: 453-458.
Vaughan HE, Jamilena M, Ruiz Rejón C, Parker JS, Garrido-Ramos MA (1993) Loss of nucleolar-organizer regions during polyploid evolution in Scilla autumnalis. Heredity 71: 574-580.
Viégas-Péquignot E (1992) In situ hybridization to chromosomes with biotinylated probes. In: Willernson D, Ed. In situ Hybridization: A Pratical Approach. Oxford University Press pp. 137-158.
Viñas A, Gómez C, Martínez P, Sánchez L (1996) Localization of rDNA genes in European eel (Anguilla anguilla). Genome 39: 1220-1223.
White MJD, Dennis ES, Honeycutt RL, Contreras N, Peacock WJ (1982) Cytogenetics of the parthenogenetic grasshopper Warramaba virgo and its bisexual relatives. IX. The ribosomal RNA cistrons. Chromosoma 85: 181-199.
Zhang Q, Saghai Maroof MA, Allard RW (1990) Effects on adaptedness of variations in ribosomal DNA copy number in populations of wild barley (Hordeum vulgare ssp. Spontaneum). Proc Natl Acad Sci USA 87: 8741-8745.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Araújo, S.M.S.R., Silva, C.C., Pompolo, S.G. et al. Genetic load caused by variation in the amount of rDNA in a wasp. Chromosome Res 10, 607–613 (2002). https://doi.org/10.1023/A:1020970820513
Issue Date:
DOI: https://doi.org/10.1023/A:1020970820513