Abstract
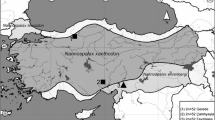
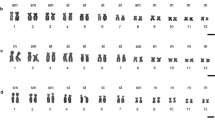
Five distinct classes of secondary constriction are found in the hylid frogs from the genera Litoria and Cyclorana, each of which is defined by its C-banding pattern and morphology (King, 1980, 1987). In-situ hybridization experiments utilizing 18S+28S copy RNA probes derived from Xenopus and Drosophila rDNA templates, were made on nine species of frogs possessing the major constriction types. Types 1, 2, 4, and 5 are confirmed as being NORs. These results also indicate that type 1 and 2 constriction types are not differentially despiralized as previously suggested, but show absolute differences in the quantity of ribosomal DNA present. This variation took two forms, deletion polymorphism and amplification polymorphism. These differences were observed between homologues within cells and between cells within individuals. Animals possessing these ‘despiralized’ constrictions are therefore mosaics for both deletion and amplification polymorphisms.
Polymorphism frequencies vary greatly between constriction types. Some specimens have a higher level of presence/absence heterozygosity, (L. moorei, type 2, L. nannotis type 5, L. raniformis) (animal A, pair 8 type 2), than do others (L. peronii, L. rothii, L. caerulea). The above species also vary markedly in the degree and frequency of amplification of the NORs. The type 4 constrictions analysed (L. coplandi, L. Lesueuri and C. novaehollandiae) have a particularly low frequency of presence/absence heterozygosity, and they have fewer size heteromorphisms between homologues. The type 3 ephemeral constrictions did not hybridize to cRNA probes at any stage.
In all but one of the species studied, a single pair of chromosomes possessed an NOR. However, in L. raniformis these occurred on two pairs of chromosomes. Each of these sites behaved independently, and the pair 8 constriction had significantly more between cell variation than did that on pair 13. The significance of these observations is discussed and is related to the evolution of NOR structure and the distribution of heterochromatin in amphibians.
Similar content being viewed by others
References
Batistoni, R., Andronico, F., Nardi, I. & Barsacchi-Pilone, G., 1978. Chromosome location of the ribosomal genes in Triturus vulgaris meridionalis (Amphibia Urodela). III. Inheritance of chromosomal sites for 18S+28S Ribosomal RNA. Chromosoma 65: 231–240.
Bickham, J. W. & Rogers, D. S., 1985. Structure and variation of the nucleolus organizer region in turtles. Genetica 67: 171–184.
Botchan, P. M., Reeder, R. A. & Dawid, I. B., 1977. Restriction analysis of the nontranscribed spacers of Xenopus laevis ribosomal DNA. Cell 11: 599.
Buys, C. H. C. M. & Osinga, J., 1980. Abundance of protein-bound sulfhydril and disulphide groups at chromosomal nucleolus organizing regions. A cytochemical study on the selective silver staining of NOR's. Chromosoma 77: 1–11.
Cabrero, J., Navas-Castillo, J. & Camacho, J. P. M., 1986. Effects of Supernumerary chromosome segments on the activity of Nucleolar organizer regions in the grasshopper Chorthippus binotatus. Chromosoma 93: 375–380.
Hennen, S., Mizuno, S. & MacGregor, H. C., 1975. In-situ hybridization of ribosomal DNA labelled with 125 Iodine to Metaphase and lampbrush chromosomes from newts. Chromosoma 50: 349–369.
Hutchison, N. & Pardue, M. L., 1975. The mitotic chromosomes of Notophthalmus (= Triturus) viridescens: Localization of C-banding regions and DNA sequences Complementary to 18S, 28S and 5S Ribosomal RNA. Chromosoma 53: 51–69.
John, B. & King, M., 1980. Heterochromatin variation in Cryptobothrus chrysophorus. III. Synthetic hybrids. Chromosoma 88: 57–68.
John, B. & King, M., 1982. Meiotic effects of supernumerary heterochromatin in Heteropternis obscurella. Chromosoma 85: 39–65.
John, B. & King, M., 1985. The inter-relationship between heterochromatin distribution and chiasma distribution. Genetica 66: 183–194.
Kezer, J. & MacGregor, H. C., 1973. The nucleolar organizer of Plethodon cinereus cinereus (Green). II. The lampbrush nucleolar organizer. Chromosoma 42: 427–444.
King, M., 1980. C-banding studies on Australian hylid frogs: secondary constriction structure and the concept of euchromatin transformation. Chromosoma 80: 191–217.
King, M., 1981. A cytotaxonomic analysis of Australian hylid frogs of the genus Litoria. Proc. herp. Symp. Melb. 169–175.
King, M., 1985. Chromosome markers and their use in phylogeny and systematics. In ‘Biology of Australasian frogs and reptiles’. Ed G. Grigg, R. Shine & H. Ehmann. Surrey Beatty and Sons. Sydney. pp. 165–175.
King, M., 1988. The inter-relationship of G-banding, C-banding pattern and Nucleolus organizer structure in anuran Amphibians. Kew Chrom. Conf. III, HMSO, pp. 51–63.
King, M. (in press): ‘Animal cytogenetics’ 4, Chordata 2, Amphibia. Borntraeger. Stuttgart, Berlin.
King, M. & King, D., 1975. Chromosomal evolution in the lizard genus Varanus (Reptilia). Aust. J. biol. Sci. 28: 89–108.
King, M., Honeycutt, R. & Contreras, N., 1986. Chromosomal repatterning in Crocodiles: C, G and N-banding and the in-situ hybridization of 18S+26S rRNA cistrons. Genetica 70: 191–201.
Macgregor, H. C. & Kezer, J., 1973. The nucleolar organizer of Plethodon cinereus cinereus (Green) I. Location of the nucleolus organizer by in-situ nucleic acid hybridization. Chromosoma 42: 415–426.
Macgregor, H. C., Vlad, M. & Barnett, L., 1977. An investigation of some problems concerning nucleolus organizers in Salamanders. Chromosoma 59: 283–299.
Macgregor, H. C. & Mizumo, S., 1976. In-situ hybridization of ‘nick translated’ 3H-ribosomal DNA to chromosomes from salamanders. Chromosoma 54: 15–25.
Mahony, M. J. & Robinson, E. S., 1986. Nucleolar organizer region (NOR) location in karyotypes of Australian ground frogs (family Myobatrachidae). Genetica 86: 119–127.
Miller, L. & Brown, D. D., 1969. Variation in the activity of nucleolus organizers and their ribosomal gene content. Chromosoma 28: 430–444.
Morgan, G. T., 1978. Absence of chiasmata from the heteromorphic region of chromosome I during spermatogensis in Triturus cristatus carnifex. Chromosoma 66: 269–280.
Morgan, G. T., Macgregor, H. C. & Colman, A., 1980. Multiple ribosomal gene sites revealed by in-situ hybridization of Xenopus rDNA to Triturus lampbrush chromosomes. Chromosoma 80: 309–330.
Nardi, I., Barsacchi-Pilone, G., Batistoni, R. & Andronico, F., 1977. Chromosome location of the ribosomal RNA genes in Triturus vulgaris meridionalis (Amphibia, Urodela). II. Intraspecific variability in number and position of the chromosome loci for 18S+28S ribosomal RNA. Chromosoma 64: 67–84.
Nardi, I., De Lucchini, S., Barsacchi-Pilone, G. & Andronico, F., 1978. Chromosome location of the ribosomal RNA genes in Triturus vulgaris meridionalis (Amphibia-Urodela) IV. Comparison between in-situ hybridization with 3H 18S+28S rRNA and AS-SAT Staining. Chromosoma 70: 91–99.
Old, R. W., Callan, H. G. & Gross, K. W., 1977. Localization of Histone gene transcripts in Newt lampbrush chromosomes by in-situ hybridization. J. Cell Sci. 27: 57–79.
Pardue, M. L., 1974. Localization of repeated DNA sequences in Xenopus chromosomes. Cold Spr. Harb. Symp. quant. Biol. 38: 475–482.
Peacock, W. J., Lohe, A., Gerlach, W. L., Dunsmuir, P., Dennis, E. S. & Appels, R., 1978. Fine structure and evolution of DNA in heterochromatin. Cold Spr. Harb. Symp. quant. Biol. 42: 1121–1135.
Scheer, U., Franke, W. W., Trendelenburg, M. F. & Spring, H., 1976. Classification of loops of lampbrush chromosomes according to the arrangement of transcriptional complexes. J. Cell Sci. 22: 503–519.
Schmid, M., 1978a. Chromosome banding in Amphibia I. Constitutive heterochromatin and nucleolus organizer regions in Bufo and Hyla. Chromosoma 66: 361–388.
Schmid, M., 1978b. Chromosome banding in Amphibia. II. Constitutive heterochromatin and nucleolus organizer regions in Ranidae, Microhylidae and Rhacophoridae. Chromosoma 68: 131–148.
Schmid, M., 1980. Chromosome banding in Amphibia. IV. Differentiation of GC-and AT-rich chromosome regions in Anura. Chromosoma 77: 83–103.
Schmid, M., 1982. Chromosome banding in Amphibia. VII. Analysis of the structure and variability of NORs in Anura. Chromosoma 87: 327–344.
Schubert, I. & Wobus, U., 1985. In-situ hybridization confirms jumping nucleolus organizing regions in Allium, Chromosoma 92: 143–148.
Sekiya, K. & Nakagawa, H., 1983. Cytogenetics of Xenopus laevis. I. G-banding pattern of Xenopus laevis chromosomes. Experientia 39: 786–787.
Stock, A. D., 1984. The occurrence of G-bands in the mitotic chromosomes of the amphiblan Xenopus muelleri. Genetica 64: 225–228.
Varley, J. M. & Morgan, G. T., 1978. Silver staining of the lampbrush chromosomes of Triturus critatus carnifex. Chromosoma 67: 233–244.
Vitelli, L., Batistoni, R., Andronico, F., Nardi, I. & Barsacchi-Pilone, G., 1982. Chromosomal localization of 18S+28S and 5S Ribosomal RNA genes in evolutionary diverse anuran Amphibians. Chromosoma 84: 475–491.
White, M. J. D., 1978. Modes of speciation. Freeman, San Francisco.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
King, M., Contreras, N. & Honeycutt, R.L. Variation within and between nucleolar organizer regions in Australian hylid frogs (Anura) shown by 18S+28S in-situ hybridization. Genetica 80, 17–29 (1990). https://doi.org/10.1007/BF00120116
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00120116